��Ŀ����
10��ijͬѧȡij��ʯ��ʯ��Ʒ12g���вⶨʵ�飬�ֽ�100gϡ�������μ���ʯ��ʯ��Ʒ�У����ʲ�����ˮҲ�����뷴Ӧ������ַ�Ӧ����������������������±���ʾ��| ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
| ����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
| ���������������/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
��1��m��ֵΪ3.3g
��2��12gʯ��ʯ��Ʒ��̼��Ƶ���������10g
��3�����������в��뷴Ӧ��ϡ������Һ�����ʵ�����Ϊ���٣�
���� ̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ݱ����ṩ�����ݺͷ�Ӧ�Ļ�ѧ����ʽ���ý�����ط���ļ�����жϣ�
��� �⣺��1������μ��������ǰ���Σ�ÿ�ζ�����1.1g������̼�����ġ��嶼����4.4g������̼��˵��������û����ȫ��Ӧ��Ҳ����1.1g������mֵ��3.3g��
��2������Ʒ��̼��Ƶ�����Ϊx��ϡ������Һ�����ʵ�����Ϊy��������Ŀ���ṩ����Ϣ��֪��̼��������ᷴӦ��ȫʱ�����ɵĶ�����̼������Ϊ4.4g
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73 44
x y 4.4g
$\frac{100}{x}$=$\frac{73}{y}$=$\frac{44}{4.4g}$
x=10g
y=7.3g
��3��ϡ������Һ�����ʵ�����Ϊ7.3g��
�ʴ�Ϊ����1��3.3��
��2��10��
��3��7.3��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�������ͬʱ�����˷������ݵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
20�����飨CH4����ˮ��Ӧ������ˮú����������壩���䷴Ӧ����ʾ��ͼ���£�

����������ʾ��ͼ�ó��Ľ�����ȷ���ǣ�������

����������ʾ��ͼ�ó��Ľ�����ȷ���ǣ�������
| A�� | ˮú���ijɷ���һ����̼������ | |
| B�� | ��Ӧǰ���Ԫ�صĻ��ϼ۾����� | |
| C�� | �÷�Ӧ�к���Ԫ�صĻ�������3�� | |
| D�� | �÷�Ӧ��ѧ����ʽ�м����ˮ�ļ�����֮��Ϊ1��1 |
1������ʵ������������ȷ���ǣ�������
| A�� | ��˿�ڿ����о���ȼ�գ��������� | B�� | NaOH����ˮ���¶��������� | ||
| C�� | ��̪�е��백ˮ����Һ��Ϊ��ɫ | D�� | ��ȼ�ճ���������ۣ�������ը |
18����ʵ�����м������и������ʵķ����������۶���ȷ���ǣ�������
| ѡ�� | ���������� | ������������ |
| A | ����˿����˿ | ���ȣ����ۻ����DZ���˿ |
| B | Ӳˮ����ˮ | �������ˮ������ĭ�����Ӳˮ |
| C | ������̼�� | ����ɫ������ɫ�������� |
| D | ʳ��ˮ������ˮ | ��ζ��������ζ����ʳ��ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
5�����������У���ȷ���ǣ�������
| A�� | ��Һ������ɫ����Һ�壬���Ȼ�����Һ | |
| B�� | ���ʵ��ܽ����ͨ��������������ı仯����������������ˮ���� | |
| C�� | ������Һʱ�������������������ʵ��ܽ�� | |
| D�� | 60��ʱ����ص��ܽ��Ϊ110g������Һ����������Һ��������Ϊ11��21 |
15������ͼ������ȷ��ӳ��Ӧ�仯��ϵ���ǣ�������
| A�� | 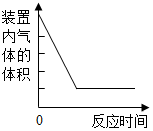 �ⶨ�����������ĺ��� | |
| B�� | 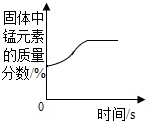 ����һ�����ĸ�����ع��� | |
| C�� | 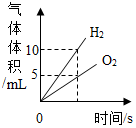 ��ˮֱͨ����һ��ʱ�� | |
| D�� | 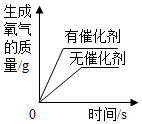 �õ���������Ũ�ȵ�˫��ˮ��ȡ���� |
13������֪ʶ���ɣ���ȫ��ȷ��һ���ǣ�������
| A����Դ���� | B����ʶ���� |
| ��̫���ܡ������������Դ �ڻ�ʯȼ���Dz���������Դ ����Ȼ��������ȡ֮��������֮���� | ��ԭ�ӱȷ���С �ڷ����˶�ԭ�Ӳ��˶� �۷�����ԭ�ӹ��� |
| C���������� | D����������; |
| �ٿ��������ձ�����Ⱦָ����Ҫ�ǵ�����������Ϳ���������� ��ˮ��Ⱦ��Ҫ���Թ�ҵ��ũҵ��������Ⱦ ��ʹ�û�ʯȼ�ϻ�Ի�����ɲ���Ӱ�� | ��CO�ж��ԣ�������ұ������ ����������ȼ������ȼ�� �����е����ԣ����������� |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |