��Ŀ����
ΪʡҩƷ��ʱ�䣬�ס��ҡ�����λͬѧ��ͭƬ��пƬ��ϡ���ᡢCuSO4��Һ(ֱ����Դ��ʯī�缫�����ߡ��ձ����Թܵ���ѧ��ѧ������ҩƷ��������Ʒ)������Ĺ�˼���������ķ�ʽ������˱Ƚ�ͭ��п����������ǿ����ϵ��ʵ�顣����д���пհף�
(1)��ͬѧ�ֱ�һСƬͭƬ��пƬ�����ձ��ײ�(ͭ��п���Ӵ�)��С�����ձ��м���ϡ���ᣬ�۲쵽��������___________________________________________________
________________________________________________________________________��
��ͬѧ�����˼·��__________________________________________________
________________________________________________________________________��
(2)��ͬѧ���ż�ʵ�飬���ձ��еμ�________��Һ�������۲쵽��������________________________________________________________________________
________________________________________________________________________��
��ͬѧ�жϳ�п��ͭ����������ǿ���������ݵ�ԭ����
________________________________________________________________________
________________________________________________________________________��
(3)��ͬѧʹ��ֱ����Դ��ʯī�缫��װ�õ��װ�ã�����ͬѧʵ������Һ�в����˱�Ҫ���Լ�(��Ϊ���Һ)����Ӧ�ڵ������漴��ʼ��ʵ�����йصĻ�ѧ��Ӧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
��õ�ʵ������������_______________________________________________
________________________________________________________________________��
(4)�����ٵ������һ����ʵ��(�Լ���������ѡ)��̽����֤ʵп��ͭ�Ľ�����Ե����ǿ��(��Ҫ˵������������)________________________________________________
________________________________________________________________________��
�𰸡�(1)пƬ�������ݲ�����ͭƬ�������ݡ�п���û������е����ͭ����
(2)CuSO4��пƬ���к�ɫ����������пƬ�ϲ������ݵ��������Լӿ졡���ý����ɰѲ����ý�����������Һ���û�������Zn��Cu��ϡ���ṹ��ԭ��أ�ZnΪ����
(3)2CuSO4��2H2O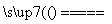 2Cu��O2����2H2SO4���������к�ɫͭ�����������������ݲ���
2Cu��O2����2H2SO4���������к�ɫͭ�����������������ݲ���
(4)�ֱ�ȡһСƬͭƬ��пƬ����֧�Թ��У����Թ��зֱ������������ɫFeCl2��Һ���Դ�Ƭ�̣���пƬ���Թ�����Һ����ɫ��ȥ����Һ��������ɫ������ͭƬ���Թ�����Һ�ޱ仯
�������ȽϽ�����Ե�ǿ�����Դ����¼����Ƕȷ������������ˮ��Ӧ�û���������׳̶ȣ��������û���Ӧ�����γ�ԭ���ʱ�ĵ缫��Ӧ���ܵ��ʱ���ӵķŵ�˳��ȡ����Լ�ͬѧ��ͭ��п��ϡ���ᷴӦ���������ų��Ƚ����߽�����Ե����ǿ������ͬѧ���ձ��м���CuSO4��Һ��п�û���ͭ������пͭԭ��أ���ͬѧ����ͬѧ�Ļ��������õ��װ�����Ƚ�ͭ��п�Ľ�����ǿ������Cu2����������ǿ��Zn2����������ͭ����⣻�������Ϸ����⣬����ͨ����ͬһ��������Ӧ���������жϻ�ԭ�Ե�ǿ�������Լ��������Ȼ�������Һ�����ݷ�Ӧ������жϽ������ǿ����

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д���֪�ϳɰ���Ӧ��Ũ���������£�
| �� N2����3H2 | |||
| ��ʼŨ��(mol/L) | 1.0 | 3.0 | 0 |
| 2 sĩŨ��(mol/L) | 0.6 | 1.8 | 0.8 |
���ð���Ũ�ȵ���������ʾ�û�ѧ��Ӧ������ʱ��������Ϊ
(����)��
A��0.2 mol/(L��s) B��0.4 mol/(L��s)
C��0.6 mol/(L��s) D��0.8 mol/(L��s)
 ���е������ס��ҡ������ֽ������ֱ����������������������ͬ������ϡ�����У�������
���е������ס��ҡ������ֽ������ֱ����������������������ͬ������ϡ�����У������� ���������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����֪�ס��ҡ������������л��ϼ۾�Ϊ+2�ۣ���������˵������ȷ���� �� ��
���������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����֪�ס��ҡ������������л��ϼ۾�Ϊ+2�ۣ���������˵������ȷ���� �� ��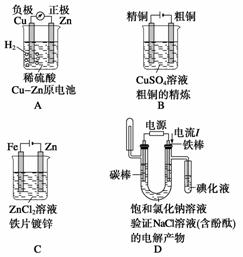

 2NH3
2NH3