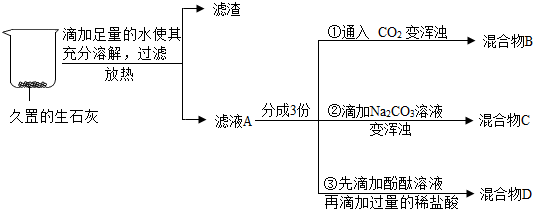
��Ŀ����
9��ˮ����Ҫ����Դ��
��1����ͼ1Ϊ���ˮ��װ�ã�A�Թ��е�������������ͨ����ʵ��֤��ˮ������Ԫ�غ���Ԫ����ɵģ�
��2����ͼ2������غ��Ȼ��Ƶ��ܽ�����ߣ�
��ͼ��a���ʾ�ĺ�����t��ʱ��KNO3��NaCl���ܽ����ȣ�
��20�棬��100gˮ�м���50g KNO3������ܽ⣮��Ҫ��߸���Һ�����ʵ������������䷽���������¶ȣ�
��3��20�棬��ͼ3ʵ��õ�����Һ�У����ʺ��ܼ�����������117��693����13��77����
���� ��1�����ݵ��ˮ��ʵ����з���������Դ�����������Թ��в�������϶࣬�����������Դ�����������Թ��в���������٣���������ͨ����ʵ��֤��ˮ������Ԫ�غ���Ԫ����ɵģ�
��2���ٸ����ܽ�����ߵĺ�����������
��20��ʱ������ص��ܽ����31.6g����100gˮ�м���50g KNO3��������ҺΪ������Һ���ҵײ���δ�ܵ�����أ��������ص��ܽ�����¶ȵı仯���Ʒ�����
��3�����ݻ�ѧ����ʽ�����������Ƶ���������Ȼ��Ƶ����������÷�Ӧ����Һ��������ȥ�Ȼ��Ƶ�������õ��ܼ��������������������ȣ�
��� ��5�֣�
�⣺��1�����ݵ��ˮ��ʵ����з���������Դ�����������Թ��в�������϶࣬�����������Դ�����������Թ��в���������٣���������ͨ����ʵ��֤��ˮ������Ԫ�غ���Ԫ����ɵģ�
��2����ͼ��a���ʾ�ĺ����ǣ�t��ʱ��KNO3��NaCl���ܽ����ȣ�
��20��ʱ������ص��ܽ����31.6g����100gˮ�м���50g KNO3��������ҺΪ������Һ���ҵײ���δ�ܵ�����أ�����ص��ܽ�����¶ȵ����߶����ߣ���ˣ���Ҫ��߸���Һ�����ʵ��������������������¶ȵķ�����ʹδ�ܵ�������ܽ⣻
��3���������Ȼ��Ƶ�����Ϊx
NaOH+HCl=NaCl+H2O
40 58.5
8g x
$\frac{40}{8g}$=$\frac{58.5}{x}$
��ã�x=11.7g
ˮ������Ϊ��8g+73g-11.7g=69.3g
���ʺ��ܼ����������ǣ�11.7g��69.3g=117��693=13��77��
�ʴ�Ϊ����1����������Ԫ�غ���Ԫ�أ���2����t��ʱ��KNO3��NaCl���ܽ����ȣ��������¶ȣ���3��117��693����13��77����
���� ������Ҫ������ˮ���顢�ܽ���������ݻ�ѧ����ʽ�ļ�������ݣ��ѶȽϴ�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | 84��85��79 | B�� | 1��1��1 | C�� | 23��24��18 | D�� | 1��1��5 |
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
| A�� | NaOH | B�� | MgCl2 | C�� | Na2CO3 | D�� | CaO |