��Ŀ����
(10��)ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8��������������Һ��������ⶨijϡ���������ʵ�����������
��1������200g��������Ϊ8��������������Һ��
�ټ��㣺��Ҫ�������ƹ��������Ϊ g��ˮ�����Ϊ mL
(ˮ���ܶȽ��ƿ���1g��cm3)��
�ڳ���������������ƽƽ�⣬��һ���ձ�����������ƽ�� �̣�������������Ȼ�� (���������Ⱥ�˳��ѡ����ĸ)��ֱ����ƽƽ�⡣
A�����������ƹ�������ձ��� B������Ҫ�������롢�ƶ�����
�ò��������ձ�������ֽ�����������Ƶ�ԭ���� ��
���ܽ⣺����Ͳ��ȡ�����ˮ������ʢ���������ƹ�����ձ�����裬ʹ���ܽ⣬����ȴ�����¡�
�ܰ���õ���Һװ���Լ�ƿ��������Ƥ�������ϱ�ǩ��
(2)��ͼ��ʾ���������Ƶ�����������Һ��20 gijϡ���ᷢ����Ӧ����Һ�¶ȵı仯�����
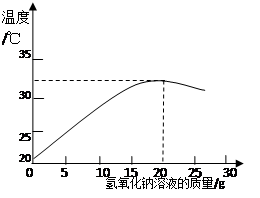
�ٸ��������жϣ�����������������Һ������Ϊ ʱ����Ӧǡ����ȫ���С�
�ڵ�����15 g����������Һʱ��������Һ�е�����Ϊ (д��ѧʽ)��
���Լ����ϡ���������ʵ���������(��д��������̣�
��1����16 184
�� �� BA ���������׳���(���������ƾ��и�ʴ�ԣ�
(2) ��20g ��HCl��NaCl
�۽⣺20g����������Һ��NaOH��������20g��8��=1��6g
���ϡ������HCl������ΪX
HCl + NaOH=NaCl+H20
36.5 40
X 1.6g
X=1.46g
ϡ�����������������100��=7��3��
����:��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�| ʵ����� | 1 | 2 | 3 | 4 | 5 |
| ϡ������ۼƼ����� | 5.0g | 10.0g | 15.0g | 20.0g | 25.0 g |
| ʣ���������� | 1.5g | 1.0g | 0.5g | 0.3g | 0.3g |
��1��2.0gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ ��ʯ��ʯ��̼��Ƶ���������Ϊ ��
��2������������㷴Ӧ������Һ���Ȼ��Ƶ�����������������̺ͽ��������һλС����
��10�֣�ij��ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ�������������ϡ������뵽20 g����ʯ�У������ɷֲ������ᷴӦ)���Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����2������NaOH��Һ���ӵ�������������±���ʾ��
| ʱ�䣯s | O | 20 | 40 | 60 | 80 | 100 | 120 |
| ���ӵ�������g | O | 3.O | 5.O | 6.O | 6.6 | 6.6 | 6.6 |
�Իش��������⣺
(1)�����ұߵ�����ֽ�ϣ��Է�Ӧʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ���ߣ�

(2)�ӱ��п��Կ�����20 g����ʯ��Ʒ�����ᷴӦ���ɵ�CO2��������� g��
(3)�������ʯ��Ʒ��̼��Ƶ�����������
��10�֣�ij��ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ�������������ϡ������뵽20 g����ʯ�У������ɷֲ������ᷴӦ)���Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����2������NaOH��Һ���ӵ�������������±���ʾ��
|
ʱ�䣯s |
O |
20 |
40 |
60 |
80 |
100 |
120 |
|
���ӵ�������g |
O |
3.O |
5.O |
6.O |
6.6 |
6.6 |
6.6 |
�Իش��������⣺
(1)�����ұߵ�����ֽ�ϣ��Է�Ӧʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ���ߣ�

(2)�ӱ��п��Կ�����20 g����ʯ��Ʒ�����ᷴӦ���ɵ�CO2��������� g��
(3)�������ʯ��Ʒ��̼��Ƶ�����������
��10�֣�ij��ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ�������������ϡ������뵽20 g����ʯ�У������ɷֲ������ᷴӦ)���Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����2������NaOH��Һ���ӵ�������������±���ʾ��
|
ʱ�䣯s |
O |
20 |
40 |
60 |
80 |
100 |
120 |
|
���ӵ�������g |
O |
3.O |
5.O |
6.O |
6.6 |
6.6 |
6.6 |
�Իش��������⣺
(1)�����ұߵ�����ֽ�ϣ��Է�Ӧʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ���ߣ�

(2)�ӱ��п��Կ�����20 g����ʯ��Ʒ�����ᷴӦ���ɵ�CO2��������� g��
(3)�������ʯ��Ʒ��̼��Ƶ�����������
