��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�˲ⶨij��������̼��Ƶ�����������С��ͬѧ����������ʵ�飬��������ϴ�������ﲢ�����ȡ10g�����ձ��Ȼ�����ձ��м���������ϡ����90g����ַ�Ӧ�Ƶ÷�Ӧʣ����Ϊ96.48g���������������ʲ������ᷴӦ����
��1������������̼�����������
��2����10g��������̼��Ƶ�������
��3���ü�������̼��Ƶ�����������
��1������������̼�����������
��2����10g��������̼��Ƶ�������
��3���ü�������̼��Ƶ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1����Ӧ��ʣ������������ȷ�Ӧǰ�ļ��٣����������غ㶨��֪�����ٵ������������ɶ�����̼��������
��2���ɶ�����̼����������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ������������̼��Ƶ�������
��3������̼��Ƶ������������̼��Ƶ������������ɣ�
��2���ɶ�����̼����������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ������������̼��Ƶ�������
��3������̼��Ƶ������������̼��Ƶ������������ɣ�
����⣨1�����������غ㶨�ɿɵã�CO2������Ϊ=10g+90g-96.48g=3.52g��
��2���輦������̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.52 g
=
x=8g
��3���ü�������̼��Ƶ���������=
��100%=80%
�𣺣�1������������̼�����������3.52g����2���ü�������̼��Ƶ�������8g����3���ü�������̼��Ƶ���������Ϊ80%��
��2���輦������̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.52 g
| 100 |
| x |
| 44 |
| 3.52g |
x=8g
��3���ü�������̼��Ƶ���������=
| 8g |
| 10g |
�𣺣�1������������̼�����������3.52g����2���ü�������̼��Ƶ�������8g����3���ü�������̼��Ƶ���������Ϊ80%��
������������Ҫ���������غ㶨�ɺͺ��������ʵĻ�ѧ����ʽ���㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
����ɢ�������˵���ζ�������ŵ���ζ��ԭ���ǣ�������
| A�����Ӽ��м�� |
| B�����ӿɷ� |
| C�����������С |
| D�����Ӳ��ϵ��˶� |
�����������ڴ�������ǣ�������
| A������ | B����Ȫˮ | C��ʯ��ʯ | D��þ |
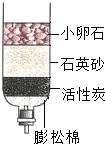 ���Ƶļ���ˮ����ͼ��ʾ���ɽ����ǵĺ�ˮ�����ˮ������ϸɳ�������ǣ�������
���Ƶļ���ˮ����ͼ��ʾ���ɽ����ǵĺ�ˮ�����ˮ������ϸɳ�������ǣ�������| A������ | B������ | C������ | D������ |
���л������У���Ԫ�ص�����������ߵ��ǣ�������
| A��FeCl2 |
| B��FeO |
| C��Fe2O3 |
| D��Fe3O4 |