��Ŀ����
ʵ������һƿ�����Ƶ�ϡ���ᣨHCl��������ˮ�õ��Ļ����Ϊϡ���ᣩ�����ǩ�IJ���������ͼ��ʾ��

��1�������ϡ��������Ԫ�ص�����������
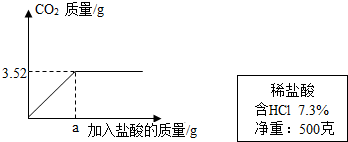
��2��ijʵ��С��������ϡ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʵ������ǣ���ȡ10gʯ��ʯ��Ʒ�гɷ�ĩ�������м���7.3����ϡ���ᣬ����������̼����������������������ϵ��ͼ��ʾ����������Ʒ�е����ʶ��������ᷴӦ������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��1��7.1%����2��80%
��������
�����������1��Ҫ�����ϡ��������Ԫ�ص�������������Ҫ�ȼ����HCl��ClԪ�ص�����������������ϡ������HCl�����������������ϡ��������Ԫ�ص�����������
�ʸ�ϡ��������Ԫ�ص���������= ��100%=7.1%
��100%=7.1%
��2���������⣬ʯ��ʯ��Ʒ��ϡ���ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽΪCaCO3+ 2HCl=CaCl2+H2O+CO2�����ٽ��ͼ���֪����ʯ��ʯ��Ʒ��ȫ��Ӧʱ�����ɵĶ�����̼����Ϊ3.52g�����û�ѧ����ʽ��̼����������̼�������ȣ���϶�����̼���������������Ʒ��̼��Ƶ�������
�⣬��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+ 2HCl=CaCl2+H2O+CO2��
100 44
x 3.52g
 =
=
��ã�x ="8g"
���ԣ�ʯ��ʯ��Ʒ��̼��Ƶ���������= ��100%��80%
��100%��80%
��ʯ��ʯ��Ʒ��̼��Ƶ�����������80%
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
�����������ǹ��ڻ�ѧ����ʽ�ļ����⣬Ҫ��ѧ���н�ǿ��ʶͼ���������ݷ�������������Ĺؼ��Ǹ���ͼ���ҳ�ǡ����ȫ��Ӧʱ�����ɵĶ�����̼�������������������صĻ�ѧ��Ӧ����������֪����δ֪��Ӧ�������������㼴�ɡ�