��Ŀ����
ʵ������һƿ�����Ƶ�ϡ���ᣨHCl��������ˮ�õ��Ļ����Ϊϡ���ᣩ�����ǩ�IJ���������ͼ��ʾ��
��1�������ϡ��������Ԫ�ص�����������
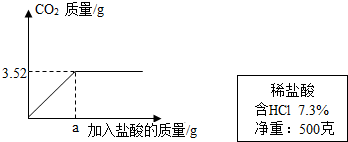
��2��ijʵ��С��������ϡ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʵ������ǣ���ȡ10gʯ��ʯ��Ʒ�гɷ�ĩ�������м���7.3����ϡ���ᣬ����������̼����������������������ϵ��ͼ��ʾ����������Ʒ�е����ʶ��������ᷴӦ������ʯ��ʯ��Ʒ��̼��Ƶ�����������


�⣺��1��(2�֣�500gX7.3%X35.5/36.5/500g=7.1��
��2����4�֣���ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx ···················· 0.5��
CaCO3+ 2HCl==CaCl2+H2O+CO2��···················· 1��
100 44
x 3.52g
 =
=
x = =8g························· 1��
=8g························· 1��
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ�� ��100%��80%··············1��
��100%��80%··············1��
��ʯ��ʯ��Ʒ��̼��Ƶ�����������80%··············0.5��

��ϰ��ϵ�д�
�����Ŀ