��Ŀ����
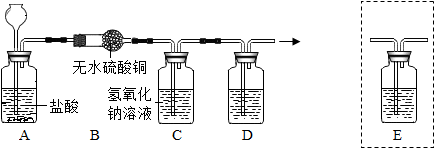
11��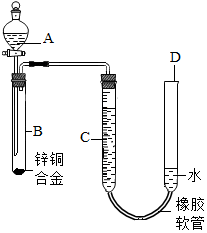 ijѧϰС������ͼװ�òⶨͭп�Ͻ���п��ͭ������������
ijѧϰС������ͼװ�òⶨͭп�Ͻ���п��ͭ��������������1����Ҫʵ����������У���������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú��ٽ��еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ�������������˳���Ǣ٢ܢۢڣ�����ţ���
��2����ʵ����ͭп�Ͻ������Ϊag�������ַ�Ӧ����������ΪV L��Ϊ����Ͻ���п��ͭ��������������ȱ�ٵ�һ��������D������ĸ����
A����Ӧǰ����ϡ������ B����Ӧǰ����ϡ�����������
C��ʵ��ǰ��Ӧװ���п�������� D��ʵ���������������ܶ�
��3����ʵ����ͭп�Ͻ������Ϊag�������ַ�Ӧ��B��ʣ����������Ϊbg����п����������Ϊ$\frac{a-b}{a}$��100%
��4��ʵ������У���δϴ�ӹ������õIJ������õ�п������������ƫС���ƫ����ƫС������Ӱ�족����
���� ��1��Ҫ�����ſ�Һ���������ⶨ����������������������¼C��Һ�棬Ȼ��ʹ��Ӧ���У�����ַ�Ӧʱ�ڼ���C��λ�ã������Զ�ʣ�������д�����
��2��Ҫ������������������֪�����������������Ҫ֪���������ܶȣ�
��3��ͭп�Ͻ������Ϊag��B��ʣ����������Ϊbg����п������Ϊ��a-b��g���Ӷ��������п������������
��4��δϴ�ӹ������õIJ�����ᵼ��ͭ������ƫ��
��� �⣺��1��Ҫ�����ſ�Һ���������ⶨ����������������������¼C��Һ�棬Ȼ��ʹ��Ӧ���У�����ַ�Ӧʱ�ڼ���C��λ�ã������Զ�ʣ�������д��������Բ�����˳��Ϊ���٢ܢۢڣ�
��2��Ҫ������������������֪�����������������Ҫ֪���������ܶȣ���ѡ��D��
��3��ͭп�Ͻ������Ϊag��B��ʣ����������Ϊbg����п������Ϊ��a-b��g���Ӷ��������п����������Ϊ��$\frac{a-b}{a}$��100%��
��4��δϴ�ӹ������õIJ�����ᵼ��ͭ������ƫ�Ӷ�п������ƫС���õ�п������������ƫС��
�ʴ�Ϊ����1���٢ܢۢڣ�
��2��D��
��3��$\frac{a-b}{a}$��100%��
��4��ƫС��
���� ����̽����ͭп�Ͻ���ͭ��п�����������IJⶨ����ɴ��⣬��������ͭ��п�����ʣ���������ṩ����Ϣ���У�

�±��������г���������Һ��pH����Ҫ�ɷֻ�ѧʽ��
| ��� | �� | �� | �� | �� | �� |
| �� �� | ʳ �� | �� �� | ����ˮ | ʯ��ˮ | ������Һ |
| ��Ҫ�ɷ� | CH3COOH | C2H5OH | C12H22O11 | Ca��OH��2 | Na2CO3 |
| ��ҺpH | 3 | 7 | 7 | 11 | 8 |
���������У�������ǿ���Ǣܣ�����ţ��������Ʒ䶣ҧ���ͷ�һ�ּ������ʣ���Ϊ����֢״�����ڶ�ҧ��Ϳ�٣�����ţ�
| A�� | 25% | B�� | 20% | C�� | 12.8% | D�� | 6.4% |
| A�� | NaOH��Һ�л���Ba��OH��2��K2SO4�� | B�� | CO2�л���HCl���壨NaOH��Һ�� | ||
| C�� | CO2�л���CO�����ȵ�̿�� | D�� | Cu��NO3��2��Һ�л���AgNO3��Cu�ۣ� |
| A�� | һ����SO42-��Na+��һ������Ba2+��CO32-�����ܺ�Cl- | |
| B�� | һ����CO32-��SO42-��һ������Ba2+��Na+�����ܺ�Cl- | |
| C�� | һ����CO32-��SO42-��Na+��һ������Ba2+�����ܺ�Cl- | |
| D�� | һ����CO32-��SO42-��һ������Ba2+�����ܺ�Cl-��Na+ |
| A�� |  ��ȡ���� | B�� |  �������� �������� | C�� |  �������� | D�� |  ��֤�������� |
 �㽭��ɽ��������ȫ������������������PHΪ5.5��6.5�����������У�����PHΪ5.5���µ�����������������Ҳ����ֲ���ں������������У�Ϊ�˷��Ρ�����̿�Ҳ����ķ���������ʱ����ũҩ������Һ����ʯ�Һ�����ͭ�Ļ��Һ���������������ϻش��������⣺
�㽭��ɽ��������ȫ������������������PHΪ5.5��6.5�����������У�����PHΪ5.5���µ�����������������Ҳ����ֲ���ں������������У�Ϊ�˷��Ρ�����̿�Ҳ����ķ���������ʱ����ũҩ������Һ����ʯ�Һ�����ͭ�Ļ��Һ���������������ϻش��������⣺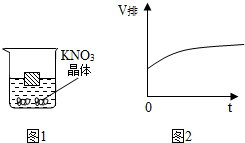 ��ͼ1��һľ��Ư����50�������ر�����Һ�У����¶ȸı�ʱ���������ɴ������ľ�����Һ����ı仯����ľ���ſ�Һ��������V������ʱ�䣨t��������ͼ2��ʾ�ı仯���ɴ��Ƴ��¶ȵĸı䷽ʽΪ���£�����¡����¡��������¶ȵĸı䣬�������Һ�����ʵ��������������٣�����䡱���������١�����
��ͼ1��һľ��Ư����50�������ر�����Һ�У����¶ȸı�ʱ���������ɴ������ľ�����Һ����ı仯����ľ���ſ�Һ��������V������ʱ�䣨t��������ͼ2��ʾ�ı仯���ɴ��Ƴ��¶ȵĸı䷽ʽΪ���£�����¡����¡��������¶ȵĸı䣬�������Һ�����ʵ��������������٣�����䡱���������١�����