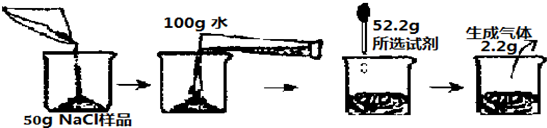
��Ŀ����
��һ�λ�ѧ�����У���ѧ��ʦҪ��ͬѧ�ǽ�30gNaCl ��Ʒ�е�����CuCl2��ȥ��ͬʱ�����������CuCl2��������������ʦ�ṩ���Լ��У�һ������������NaOH��Һ��KOH��Һ��ϡ�����100g������ͬѧ˳��������������ķ����ǣ���30g NaCl��Ʒ����100gˮ�У�����ȫ���ܽ����������ѡ�Լ�����������Լ�����Ϊ56gʱ��ǡ����ȫ��Ӧ��������ɳ���������Ϊ4.9g����ش���1������ͬѧ��ѡ�õ��Լ���
��2����NaCl��Ʒ��CuCl2�����������Ƕ��٣�
��3����ǡ����ȫ��Ӧʱ��������Һ�����ʵ����������Ƕ��٣�
��������1����ѡ������Ҫ���Ȼ�ͭ������Ӧ�������Ȼ��Ʒ�����Ӧ���ڳ�ȥ�Ȼ�ͭ��ͬʱ�����ܻ����������ʣ�
��2��д���Ȼ�ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ������������ͭ��������������Ȼ�ͭ���������ٸ���
��100%��������Ȼ�����Ʒ���Ȼ�ͭ������������
��3��������Һ�����ʵ���������=
��100%������Ϊ�Ȼ��ƣ�������Ʒ�е��Ȼ��ƣ���Ʒ������-�Ȼ�ͭ���������ͷ�Ӧ���ɵ��Ȼ��ƣ����ݳ����������������������Һ������=���ӵ��������ʵ�������-���ɳ�����������
��2��д���Ȼ�ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ������������ͭ��������������Ȼ�ͭ���������ٸ���
| �Ȼ�ͭ������ |
| �Ȼ�����Ʒ������ |
��3��������Һ�����ʵ���������=
| ���ʵ����� |
| ������Һ������ |
����⣺��1�������������Ȼ��Ʋ���Ӧ�������������Ȼ�ͭ��Ӧ����������ͭ�������Ȼ��ƣ�ʹ������������Һ�����ڳ�ȥ�Ȼ�ͭ��ͬʱ�ֵõ��������Ȼ��ƣ����Դﵽʵ��Ŀ�ģ���ʹ���������أ������������Ȼ�ͭ��Ӧ����������ͭ���Ȼ��أ��ڳ�ȥ�Ȼ�ͭ��ͬʱ�ֻ����Ȼ��أ���ʹ��ϡ���ᣬ�������Ȼ�ͭ����Ӧ���ʴ�Ϊ������������Һ
��2�����Ȼ�ͭ������Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy
CuCl2+2NaOH=2NaCl+Cu��OH��2��
135 117 98
x y 4.9g
=
=
x=6.75g y=5.85g
��100%=22.5%
�𣺸�NaCl��Ʒ��CuCl2������������22.5%��
��3��������Һ�����ʵ���������=
��100%=16.1%
��������Һ�����ʵ�����������16.1%��
��2�����Ȼ�ͭ������Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy
CuCl2+2NaOH=2NaCl+Cu��OH��2��
135 117 98
x y 4.9g
| 135 |
| x |
| 98 |
| 4.9g |
| 117 |
| y |
x=6.75g y=5.85g
| 6.75g |
| 30g |
�𣺸�NaCl��Ʒ��CuCl2������������22.5%��
��3��������Һ�����ʵ���������=
| 30g-6.75g+5.85g |
| 30g+100g+56g-4.9g |
��������Һ�����ʵ�����������16.1%��
����������һ�������ַ����������ʳ��׳�ȥ������ת��Ϊ�����ʣ���ѡ���ʲ����������ʷ�����Ӧ��

��ϰ��ϵ�д�
�����Ŀ