��Ŀ����
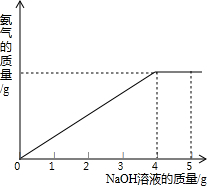 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����20%��NaOH��Һ���������·�Ӧ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O��Ӧ�����зų��İ�������������NaOH��Һ�������Ĺ�ϵ����ͼ��ʾ������㣺
��1��NH4NO3��N��H��O��������Ϊ
��2��ǡ����ȫ��Ӧ����NaOH��Һ������Ϊ
��3����Ʒ������淋�����������
��4��ǡ����ȫ��Ӧʱ������Һ��NaNO3����������������һλС������
��������1�������и�Ԫ�ص�������Ϊ��ԭ�ӵ����ԭ����������ԭ�ӵĸ����Ļ�֮�ȣ�
��2����ͼ�пɿ�������������Һ�μӵ�4��ʱ�����ǡ����ȫ��Ӧ��
��3�����ݲμӷ�Ӧ���������Ƶ������������淋�����������
��100%�����Ʒ������淋�����������
��3�����ݲμӷ�Ӧ���������Ƶ�����������ɵ������Ƶ������������ɵ������Ƶ�����+ԭ�е������Ƶ�����Ϊ������Һ�������Ƶ�����������Ʒ������+��������������Һ������-�����������������������ɸ����������Ƶ����������Ϊ������Һ���������ٸ���
��100%�������������Һ�������Ƶ�����������
��2����ͼ�пɿ�������������Һ�μӵ�4��ʱ�����ǡ����ȫ��Ӧ��
��3�����ݲμӷ�Ӧ���������Ƶ������������淋�����������
| ����淋����� |
| ��Ʒ������ |
��3�����ݲμӷ�Ӧ���������Ƶ�����������ɵ������Ƶ������������ɵ������Ƶ�����+ԭ�е������Ƶ�����Ϊ������Һ�������Ƶ�����������Ʒ������+��������������Һ������-�����������������������ɸ����������Ƶ����������Ϊ������Һ���������ٸ���
| ������Һ�������Ƶ����� |
| ������Һ������ |
����⣺��1��������е�Ԫ�ء���Ԫ�ء���Ԫ�ص�������=14��2��1��4��16��3=7��1��12
��2����ͼ��֪����������������������ʱ��˵������狀���������ǡ�÷�Ӧ������ǡ����ȫ��Ӧ����NaOH��Һ������Ϊ4g��
��3����2.0g��Ʒ��NH4NO3��������Ϊ������Ӧ����NaNO3������Ϊy����Ӧ���ɵ�NH3������Ϊz��
NH4NO3 +NaOH�TNaNO3 +NH3��+H2O
80 40 85 17
��4g��20% y z
=
x=1.6g
=
y=1.7g
=
z=0.34g
��Ʒ������淋���������=
��100%=80%
��4��������Һ�������Ƶ�����=2g-1.6g+1.7g=2.1g
������Һ������=2g+5g-0.34g=6.66g
������Һ�������Ƶ���������=
��100%=31.5%
�ʴ�Ϊ��
��1��7��1��12��
��2��4g��
��3��80%��
��4��31.5%��
��2����ͼ��֪����������������������ʱ��˵������狀���������ǡ�÷�Ӧ������ǡ����ȫ��Ӧ����NaOH��Һ������Ϊ4g��
��3����2.0g��Ʒ��NH4NO3��������Ϊ������Ӧ����NaNO3������Ϊy����Ӧ���ɵ�NH3������Ϊz��
NH4NO3 +NaOH�TNaNO3 +NH3��+H2O
80 40 85 17
��4g��20% y z
| 80 |
| x |
| 40 |
| 0.8g |
x=1.6g
| 40 |
| 0.8g |
| 85 |
| y |
y=1.7g
| 40 |
| 0.8g |
| 17 |
| z |
z=0.34g
��Ʒ������淋���������=
| 1.6g |
| 2g |
��4��������Һ�������Ƶ�����=2g-1.6g+1.7g=2.1g
������Һ������=2g+5g-0.34g=6.66g
������Һ�������Ƶ���������=
| 2.1g |
| 6.66g |
�ʴ�Ϊ��
��1��7��1��12��
��2��4g��
��3��80%��
��4��31.5%��
��������������ʱҪ��4������������Һ��5������������Һ�����壬�������ã��μӷ�Ӧ������������Һ��������4�ˣ����ӵ�����������Һ��������5�ˣ�Ȼ�������û�ѧ��Ӧ����ʽ���м��㣮

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
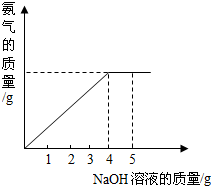 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ����ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ����ͼ��ʾ��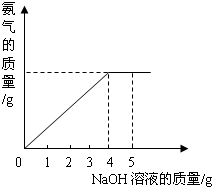 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��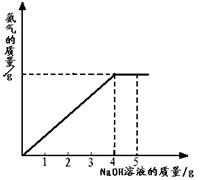 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ�� �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij�������� NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ2.0g���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��������йؼ��㣺
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij�������� NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ2.0g���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��������йؼ��㣺