��Ŀ����
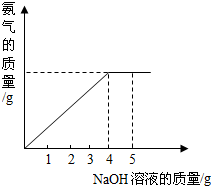
 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij�������� NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ2.0g���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��������йؼ��㣺
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij�������� NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ2.0g���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��������йؼ��㣺��1����Ʒ������淋�����������
��2��������Һ��
��
��
�ԣ�������Һ��������2.1g
2.1g
����������1�����ݹ�ϵͼ��֪����4.0g 20%������������Һʱ��ǡ����ȫ��Ӧ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����������Ƶ������ɼ�����Ʒ������淋���������Ӧ���������Ƶ�������������ʵĴ��ȵļ��㹫ʽ�������淋�����������
��2�����ڼ��������������Һ���������жϷ�Ӧ��������Һ�ʼ��ԣ�������Һ��NaNO3������Ϊ��Ʒ�������Ƶ������뷴Ӧ���������Ƶ������ͣ�
��2�����ڼ��������������Һ���������жϷ�Ӧ��������Һ�ʼ��ԣ�������Һ��NaNO3������Ϊ��Ʒ�������Ƶ������뷴Ӧ���������Ƶ������ͣ�
����⣺��1�����ݹ�ϵͼ��֪����4.0g 20%������������Һʱ��ǡ����ȫ��Ӧ����ʱ�����������Ƶ�����Ϊ��4g��20%=0.8g��
��2.0g��Ʒ�к�����淋�����Ϊ������Ӧ���������Ƶ�����Ϊy��
NH4NO3+NaOH�TNaNO3+NH3��+H2O
80 40 85
��0.8g y
=
=
����=1.6g��y=1.7g��
��Ʒ������淋���������=
��100%=80%��
��2�����ݷ�Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵͼ�����������������Һ���������������Һ�ʼ��ԣ�
��Ӧ��������Һ��NaNO3������Ϊ��1.7g+��2.0g-1.6g��=2.1g��
�𣺣�1����Ʒ������淋���������Ϊ80%��
��2��������Һ�ʼ��ԣ�������Һ��NaNO3������Ϊ2.1g��
��2.0g��Ʒ�к�����淋�����Ϊ������Ӧ���������Ƶ�����Ϊy��
NH4NO3+NaOH�TNaNO3+NH3��+H2O
80 40 85
��0.8g y
| 80 |
| x |
| 40 |
| 0.8g |
| 85 |
| y |
����=1.6g��y=1.7g��
��Ʒ������淋���������=
| 1.6g |
| 2.0g |
��2�����ݷ�Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵͼ�����������������Һ���������������Һ�ʼ��ԣ�
��Ӧ��������Һ��NaNO3������Ϊ��1.7g+��2.0g-1.6g��=2.1g��
�𣺣�1����Ʒ������淋���������Ϊ80%��
��2��������Һ�ʼ��ԣ�������Һ��NaNO3������Ϊ2.1g��
�����������Ĺؼ��Ǹ��ݷ�Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵͼ����ȷ���ߵ��۵㼴����4.0g����������Һʱǡ����ȫ��Ӧ��

��ϰ��ϵ�д�
�����Ŀ
 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ����ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ����ͼ��ʾ��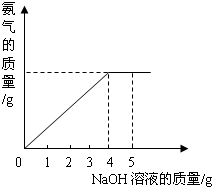 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��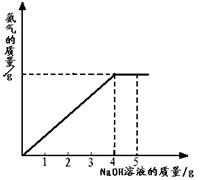 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����5.0g 20%��NaOH��Һ���������·�Ӧ��NH4NO3+NaOH=NaNO3+NH3��+H2O����Ӧ�����зų��İ��������������NaOH��Һ�������Ĺ�ϵ��ͼ��ʾ��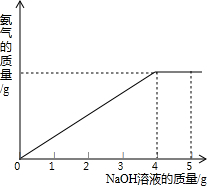 �������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����20%��NaOH��Һ���������·�Ӧ��
�������ũҵ�����г��õĻ�ѧ���ϣ�Ϊ�ⶨij��������NaNO3���������Ʒ��NH4NO3�Ĵ��ȣ�ȡ 2.0g ���������Ʒ�������У�����20%��NaOH��Һ���������·�Ӧ��