��Ŀ����
6��ͨ������ʵ������������ܵó���Ӧ���۵��ǣ�������| ʵ����� | ���� | ���� | |
| A | ��ȼ���ŵ�ľ�����ʢ��ij����ļ���ƿ�� | ��ľ������Ϩ�� | ���������Ƕ�����̼ |
| B | ��ij��ɫ��Һ�еμ�����������Һ | ���д̼�����ζ�������� | ������Һ��һ������NH4+ |
| C | ��ij��ɫ��Һ�еμ�ϡ���� | ������ɫ���� | ����ɫ��Һ��һ������ CO32-���� |
| D | ��������ͬ��þ����п�ֱ������ͬŨ�Ⱥ����������������� | ��þ�������� ����������� | þ�Ľ�����Ա�пǿ |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� ������̼��������ϡ����������ʶ�����֧��ȼ�գ�
笠������ܺ����������ӽ������ˮ�Ͱ������������д̼�����ζ��
̼����������ܺ������ӽ������ˮ�Ͷ�����̼��
�Ա�����Ҫȷ���ó����ͱ�����
��� �⣺A����ȼ���ŵ�ľ�����ʢ��ij����ļ���ƿ�У�ľ������Ϩ�𣬲���˵��������һ���Ƕ�����̼��Ҳ�����ǵ�����ϡ������ȣ���ѡ��˵������ȷ��
B����ij��ɫ��Һ�еμ�����������Һ���д̼�����ζ�������ɣ�˵������Һ��һ������笠����ӣ���ѡ��˵����ȷ��
C����ij��ɫ��Һ�еμ�ϡ���ᣬ������ɫ���壬����˵����ɫ��Һ��һ������̼������ӣ�Ҳ���ܺ���̼��������ӣ���ѡ��˵������ȷ��
D����������ͬ��þ����п�ֱ������ͬŨ�Ⱥ����������������У�þ�����������������죬����˵��þ�Ľ�����Ա�пǿ��������Ϊʹ�õ��ͬ����ѡ��˵������ȷ��
��ѡ��B��
���� ʵ������������֮������õ����ڱ��֣����Ҫѧ�����ʵ�顢�۲�ʵ�顢����ʵ�飬Ϊ��ʾ����֮������õ�ʵ�ʵ춨������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
16������ʵ������У��ܴﵽʵ��Ŀ���ǣ�������
| A�� | ������������ʱ��û�������κ����������������Ѵ��� | |
| B�� | ���������Լ���������K2SO4��Һ��Na2CO3��Һ��BaCl2��Һ��ϡHNO3 | |
| C�� | �����ᴿʵ���У������ᾧֱ����Һ����ʱֹͣ���� | |
| D�� | ��pH��ֽ�ⶨ��Һ����ʱ���Ƚ�pH��ֽ��ˮ��ʪ��Ȼ���ٲⶨ |
17�����м������ʵķ���������ǣ�������
| A�� | �÷�̪����ʳ��ˮ��ϡ���� | |
| B�� | �ö�����̼����������ʯ��ˮ�ͻ����Һ | |
| C�� | ��ʳ��������е�ʳ�κ�С�մ� | |
| D�� | �÷���ˮ����Ӳˮ����ˮ |
18�� ��У���dz���ѧ����ȤС��ͬѧ��ʵ��������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��У���dz���ѧ����ȤС��ͬѧ��ʵ��������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��������⡿��ƿ�Լ�������ʲô��Һ�أ�
���������ۡ����������ǩ������жϣ���ƿ�Լ���������A��
A���� B���� C����
���������ϡ�
���л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NaHCO3��
��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
�ⶨ���£�20�棩ʱ���������ʵ��ܽ�ȵ��������£�
���ó����ۡ�С�������Լ�ƿ��ע��������������10%���ϱ��е��ܽ�ȵ������жϣ���ƿ�Լ���������NaHCO3��
���������롿�ٿ�����NaOH��Һ���ڿ�����Na2CO3��Һ���ۿ�����NaCl��
����Ʋ�ʵ�顿
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH��7����ƿ�Լ��������Ǣۣ�
��2��СǿΪ�˼������Һ��NaOH��Һ����Na2CO3��Һ�����ֽ���������ʵ�飺
��3������ѡ����Сǿ��ͬ�����Լ���������NaOH��Һ��Na2CO3��Һ����ѡ��Ca��OH��2��Һ��
 ��У���dz���ѧ����ȤС��ͬѧ��ʵ��������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��У���dz���ѧ����ȤС��ͬѧ��ʵ��������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽������������⡿��ƿ�Լ�������ʲô��Һ�أ�
���������ۡ����������ǩ������жϣ���ƿ�Լ���������A��
A���� B���� C����
���������ϡ�
���л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NaHCO3��
��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
�ⶨ���£�20�棩ʱ���������ʵ��ܽ�ȵ��������£�
| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��/g | 36 | 109 | 215 | 9.6 |
���������롿�ٿ�����NaOH��Һ���ڿ�����Na2CO3��Һ���ۿ�����NaCl��
����Ʋ�ʵ�顿
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH��7����ƿ�Լ��������Ǣۣ�
��2��СǿΪ�˼������Һ��NaOH��Һ����Na2CO3��Һ�����ֽ���������ʵ�飺
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ��������Һ��һ�ྻ�Թ��еμ��Ȼ�����Һ | ������ɫ���� | �������ȷ ��صĻ�ѧ����ʽBaCl2+Na2CO3�TBaCO3��+2NaCl�� |
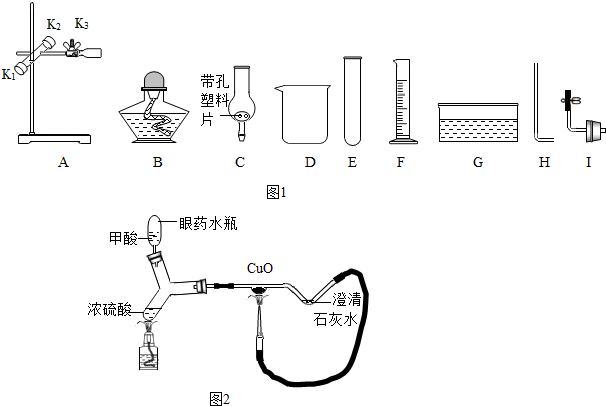

 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮