��Ŀ����
20����֪��ͬ��Ԫ����ɵIJ�ͬ�ֵ��ʣ�����Ϊͬ�������壮������ʯ��ʯī��ͬ�������壬���Ͱ���Ҳ��ͬ�������壮ijͬѧ�����й����ϵ�֪�����Ц�-Fe����-Fe����-Fe����ͬ�������壮���־����ڲ�ͬ�¶����ܷ�������ת������-Fe$\stackrel{1394��}{?}$��-Fe$\stackrel{912��}{?}$��-Fe�����й�˵����ȷ���ǣ�������
| A�� | ��-Fe����-Fe����-Fe֮����ת���ǻ�ѧ�仯 | |
| B�� | ��-Fe����-Fe����-Feһ��������ͬ��Ԫ�� | |
| C�� | ��-Fe����-Fe����-Fe����ͬ������ | |
| D�� | ��-Fe����-Fe����-Fe���������ʵ����ʶ���ͬ |
���� ���������������ͬ��������Ķ��壬����֪����νͬ���������������ʱ��ֵ���ʽ֮һ�����Ԫ����ͬ���������ʵĻ�ѧʽ����̬����״��ͬ�������������Ԫ����ͬ�������ǵĻ�ѧ��������ͬ�ģ����Ծݴ˽����⣮
��� �⣺A����-Fe����-Fe����-Fe֮����ת���ǻ�ѧ�仯����Ϊ���������ֲ�ͬ�����ʣ�����ȷ��
B����-Fe����-Fe����-Feͬ������Ԫ�أ��ʴ���
C����������ͬ�������嶼������ԭ�ӹ��ɣ�������ԭ������˳��ͬ�������������ֲ�ͬ�ĵ��ʣ��ʴ���
D����Ϊ��-Fe����-Fe����-Fe�����ֲ�ͬ�����ʣ���ԭ�ӵ����з�ʽ��ͬ�����������������ϴ����Ų��죬�ʴ���
��ѡA��
���� Ҫ�����������Ŀ�����ȣ�Ҫ��������ͬ���������ͬ����������Ȼ���������������龰�����ѧ�����֪ʶ�ͼ��ܣ���ϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�

��ϰ��ϵ�д�
 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
�����Ŀ
10���������ʵķ�����ȷ���ǣ�������
| A�� | ���ý�����þ�������� | B�� | �����ˮ������ͭ����ʯ�� | ||
| C�� | ����������ʯ�͡����� | D�� | �Σ��ռ���̼��� |
8��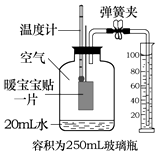 ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������
ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������
 ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������
ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������| A�� | ʵ��ǰ������װ�õ������� | |
| B�� | ����ʵ�����ݲ�ÿ������������������Ϊ18% | |
| C�� | ��ʵ���ÿ����������������ƫ�ߣ�������ů��������ʹ���������� | |
| D�� | ������¶ȼƵĶ����ָ���ʵ��ǰ���¶Ⱥ���ܼ�¼��Ͳ��ʣ��ˮ����� |
15���ճ������У���������̼������Һ���м��ԣ���ϴ�;��ϵ����ۣ�����Խǿ��ȥ���۵�Ч��Խ�ã������Ƕ�Ӱ��̼������Һ���Ե�����չ��̽����
��̼���ƹ���Ͳ�ͬ�¶ȵ�ˮ�������������������ֱ�Ϊ2%��6%��10%��̼������Һ������������Һ��pH����¼�������±���
��������������ݻش�
��1��ȥ���۵�Ч����õ��Ǣᣨ��ʵ���ţ���
��2����һ���¶ȷ�Χ�ڣ��¶ȶ�̼������ҺpH��Ӱ���ǣ���̼������Һ������������ͬʱ���¶�Խ�ߣ�pHֵԽ��
��3��Ҫ����̼������Һ��pH����Һ���������������ı仯��ϵ���ߣ���ѡ���һ��ʵ����
�٢ܢߣ���ۢޢᣨ��ʵ���ţ�����������һ��������������Χ�ڣ��¶�һ��ʱ��������������Խ����ҺpHԽ��
��̼���ƹ���Ͳ�ͬ�¶ȵ�ˮ�������������������ֱ�Ϊ2%��6%��10%��̼������Һ������������Һ��pH����¼�������±���
| ʵ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ������������ | 2% | 2% | 2% | 6% | 6% | 6%] | 10% | 10% | 10% |
| ˮ���¶ȣ��棩 | 20 | 40 | 60 | 20 | 50 | 60 | 20 | 40 | 60 |
| ��ҺpH | 10.90 | 11.18 | 11.26 | 11.08 | 11.27 | 11.30 | 11.22 | 11.46 | 11.50 |
��1��ȥ���۵�Ч����õ��Ǣᣨ��ʵ���ţ���
��2����һ���¶ȷ�Χ�ڣ��¶ȶ�̼������ҺpH��Ӱ���ǣ���̼������Һ������������ͬʱ���¶�Խ�ߣ�pHֵԽ��
��3��Ҫ����̼������Һ��pH����Һ���������������ı仯��ϵ���ߣ���ѡ���һ��ʵ����
�٢ܢߣ���ۢޢᣨ��ʵ���ţ�����������һ��������������Χ�ڣ��¶�һ��ʱ��������������Խ����ҺpHԽ��
5�����й���a��b��ֵ�ıȽ��У�aһ��С��b���ǣ�������
| A�� | ��������Һ�����ʵ���������Ϊa%��������Һ�����ʵ���������Ϊb% | |
| B�� | ͬһ������Һ�У����ʵ���������Ϊa%���ܽ��Ϊb�� | |
| C�� | ij���ʵı�����Һ�����ʵ���������Ϊa%�������м������������ʺ����ʵ���������Ϊb% | |
| D�� | ij���ʵ��ܽ���ڵ���ʱΪa�ˣ�����ʱΪb�� |
9��һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�ϣ��ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��
��1��ú����ˮ������Ӧ��������CO��H2����Ӧ�Ļ�ѧ����ʽΪC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2����Ȼ�����������Եõ��ϳ��������۹���ʾ��ͼ������ʾ��
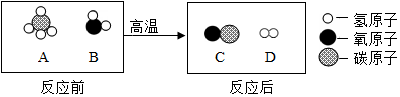
���������У�������C��D��������Ϊ14��3��
��3���ϳ�����CO��H2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ����Ժϳ���Ϊԭ�ϲ����ܵõ���������C������ĸ��ţ���
A�����ᣨH2C2O4�� B���״���CH3OH�� C������[CO��NH2��2]
��4�������±��ش�������⣮
��������������ʵ��ܽ����ȵ��¶ȷ�Χ����20��40�森
��20��ʱ������ص��ܽ����31.6g�����¶��£���20gKNO3����50gˮ�У���ֽ��裬������Һ��������65.8g��Ҫ��һ����߸���Һ�����������������ɽ��еIJ��������£�
��1��ú����ˮ������Ӧ��������CO��H2����Ӧ�Ļ�ѧ����ʽΪC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2����Ȼ�����������Եõ��ϳ��������۹���ʾ��ͼ������ʾ��

���������У�������C��D��������Ϊ14��3��
��3���ϳ�����CO��H2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ����Ժϳ���Ϊԭ�ϲ����ܵõ���������C������ĸ��ţ���
A�����ᣨH2C2O4�� B���״���CH3OH�� C������[CO��NH2��2]
��4�������±��ش�������⣮
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g/100g ˮ�� | KNO3 | 13.3 | 31.6 | 63.9 | 110.0 | 169.0 | 246.0 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 | |
��20��ʱ������ص��ܽ����31.6g�����¶��£���20gKNO3����50gˮ�У���ֽ��裬������Һ��������65.8g��Ҫ��һ����߸���Һ�����������������ɽ��еIJ��������£�