��Ŀ����
̼���仯������������Ӧ�ù㷺��
�ٽ�ʯī���������缫����һ����̼��������ȼ�ϣ��۸ɱ������˹����ꣻ��һ����̼������������
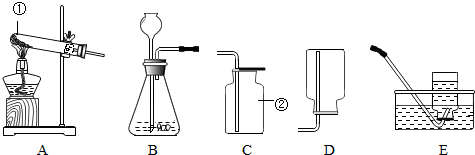
��1��������;�У���Ҫ���������ʵ��������ʵ��� ��������ţ�
��2���������⣬д��������̼��һ����; ��
��3���ɱ����������Ͳ����� ��������ţ�
A������� B�������� C�������� D��������
��4��̼��ȶ��ֽ⣬̼��ֽ�Ļ�ѧ����ʽΪ ��
��5��һ����̼ȼ��ʱ�������ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�ٽ�ʯī���������缫����һ����̼��������ȼ�ϣ��۸ɱ������˹����ꣻ��һ����̼������������
��1��������;�У���Ҫ���������ʵ��������ʵ���
��2���������⣬д��������̼��һ����;
��3���ɱ����������Ͳ�����
A������� B�������� C�������� D��������
��4��̼��ȶ��ֽ⣬̼��ֽ�Ļ�ѧ����ʽΪ
��5��һ����̼ȼ��ʱ�������ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ
���㣺̼���ʵ��������ʼ���;,������̼����;,һ����̼�Ļ�ѧ����,�������ʶ��������,������ͻ������б�,���ʺͻ�������б�,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺̼�����뺬̼���������������;
��������1�������������ʺͻ�ѧ���ʵı�������---�Ƿ�ͨ����ѧ�仯���ܱ��ֳ����жϣ�
��2�����ݶ�����̼�����ʽ��н��
��3�����ݸɱ�����̼����Ԫ����ɵĴ�������н��
��4������̼��ֽ����ɶ�����̼��ˮ���н��
��5������һ����̼ȼ�����ɶ�����̼���н��
��2�����ݶ�����̼�����ʽ��н��
��3�����ݸɱ�����̼����Ԫ����ɵĴ�������н��
��4������̼��ֽ����ɶ�����̼��ˮ���н��
��5������һ����̼ȼ�����ɶ�����̼���н��
����⣺��1��������;�У���Ҫ���������ʵ��������ʵ��н�ʯī���������缫�ɱ������˹����꣬��һ����̼��������ȼ�Ϻ�һ����̼�������������ڻ�ѧ���ʣ�
��2��������̼��ȼ��Ҳ��֧��ȼ�գ����Կ��������
��3���ɱ�����̼����Ԫ����ɵĴ�������ڻ����ͬʱҲ������������ǻ���
��4��̼��ֽ����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽ�ǣ�H2CO3�TH2O+CO2����
��5��һ����̼ȼ�����ɶ�����̼����Ӧ�Ļ�ѧ����ʽ2CO+O2
2CO2��
�ʴ�Ϊ����1���٢ۣ���2����𣻣�3��A����4��H2CO3�TH2O+CO2������5��2CO+O2
2CO2��
��2��������̼��ȼ��Ҳ��֧��ȼ�գ����Կ��������
��3���ɱ�����̼����Ԫ����ɵĴ�������ڻ����ͬʱҲ������������ǻ���
��4��̼��ֽ����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽ�ǣ�H2CO3�TH2O+CO2����
��5��һ����̼ȼ�����ɶ�����̼����Ӧ�Ļ�ѧ����ʽ2CO+O2
| ||
�ʴ�Ϊ����1���٢ۣ���2����𣻣�3��A����4��H2CO3�TH2O+CO2������5��2CO+O2
| ||
�������������ʺͻ�ѧ�����dz��л�ѧ����Ҫ�Ļ�������֮һ�������������ʺͻ�ѧ���ʵĶ��壬�����������ʺͻ�ѧ���ʵı�������----�Ƿ�ͨ����ѧ�仯���ֳ������˽���߰����������ǽ����������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
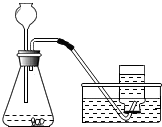 ������̼��ʵ�����Ʒ�
������̼��ʵ�����Ʒ�
 �ꡢѩ���γɺͽ�������п����ա��ܽ������SO2��������������ʣ��γ���pHС��5.6�Ľ�ˮ��Ϊ���꣮��ش������й���������⣺
�ꡢѩ���γɺͽ�������п����ա��ܽ������SO2��������������ʣ��γ���pHС��5.6�Ľ�ˮ��Ϊ���꣮��ش������й���������⣺ ���й�ˮ�ܡ������������Ϊ����չˮ����������������
���й�ˮ�ܡ������������Ϊ����չˮ����������������
