��Ŀ����
ij��ѧ��ȤС���������ʯ��̼��Ƶ��������������dz�ȡ11����Ʒ����80��ϡ�����4�μ��룬��õ��������±������ʲ������ᷴӦ��Ҳ������ˮ������ͼ������ʵ���ҳ��õ�������ȡװ�ã���ش�
��1������ʯ��̼��Ƶ�����������
��2����Ӧ���õ�ϡ���������������
��3��ǡ����ȫ��Ӧʱ���ʵ�����������
| ����ϡ����Ĵ��� | 1 | 2 | 3 | 4 |
| ����ϡ������������ˣ� | 20 | 20 | 20 | 20 |
| ʣ�������������ˣ� | 7 | 3 | 1 | 1 |
��2����Ӧ���õ�ϡ���������������
��3��ǡ����ȫ��Ӧʱ���ʵ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1����ͼʾ���ݿ�֪�������κ͵��Ĵ�ʣ������������ȣ�˵�������η�Ӧ�д���ʯ��̼�������ȫ��Ӧ��ʣ�������������Ǵ���ʯ�����ʵ��������ݴ˵ó�����ʯ��̼��Ƶ����������������ʯ��̼��Ƶ������������ɣ�
��2�����ݵ�һ����ϡ������ȫ��Ӧ����20gϡ������ȫ��Ӧ����̼��Ƶ�����Ϊ11g-7g=4g�������ι������������2g�����Ե�����ֻ��10g����μӷ�Ӧ������̼��Ƶ���������μ���Ӧ���������ʵ��������Ӷ�������õ�ϡ����������������н��
��3������ǡ����ȫ��Ӧʱ�������������Ϊ50g����������Ȼ��Ƶ�������������������������ʽ���ǡ����ȫ��Ӧʱ���ʵ������������ɣ�
��2�����ݵ�һ����ϡ������ȫ��Ӧ����20gϡ������ȫ��Ӧ����̼��Ƶ�����Ϊ11g-7g=4g�������ι������������2g�����Ե�����ֻ��10g����μӷ�Ӧ������̼��Ƶ���������μ���Ӧ���������ʵ��������Ӷ�������õ�ϡ����������������н��
��3������ǡ����ȫ��Ӧʱ�������������Ϊ50g����������Ȼ��Ƶ�������������������������ʽ���ǡ����ȫ��Ӧʱ���ʵ������������ɣ�
����⣺��1������ʯ��̼��Ƶ���������=
��100%��90.9%��
��2����һ����ϡ������ȫ��Ӧ����20gϡ������ȫ��Ӧ����̼��Ƶ�����Ϊ11g-7g=4g�������ι������������2g�����Ե�����ֻ��10g����μӷ�Ӧ�����Բμӷ�Ӧ���������=20g+20g+10g=50g��̼�������=11g-1g=10g��
�����ɶ�����̼������Ϊx���μ���Ӧ���������ʵ�����Ϊy�������Ȼ��Ƶ�����Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
10g y z x
=
x=4.4g
=
y=7.3g
=
z=11.1g
��Ӧ���õ�ϡ�������������=
��100%=14.6%
��3��ǡ����ȫ��Ӧʱ���ʵ���������=
��100%��20%
�𣺣�1������ʯ��̼��Ƶ���������Ϊ90.9%��
��2����Ӧ���õ�ϡ�������������Ϊ14.6%��
��3��ǡ����ȫ��Ӧʱ���ʵ���������Ϊ20%��
| 11g-1g |
| 11g |
��2����һ����ϡ������ȫ��Ӧ����20gϡ������ȫ��Ӧ����̼��Ƶ�����Ϊ11g-7g=4g�������ι������������2g�����Ե�����ֻ��10g����μӷ�Ӧ�����Բμӷ�Ӧ���������=20g+20g+10g=50g��̼�������=11g-1g=10g��
�����ɶ�����̼������Ϊx���μ���Ӧ���������ʵ�����Ϊy�������Ȼ��Ƶ�����Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
10g y z x
| 100 |
| 10g |
| 44 |
| x |
x=4.4g
| 100 |
| 10g |
| 73 |
| y |
y=7.3g
| 100g |
| 10g |
| 111 |
| z |
z=11.1g
��Ӧ���õ�ϡ�������������=
| 7.3g |
| 50g |
��3��ǡ����ȫ��Ӧʱ���ʵ���������=
| 11.1g |
| 10g+50g-4.4g |
�𣺣�1������ʯ��̼��Ƶ���������Ϊ90.9%��
��2����Ӧ���õ�ϡ�������������Ϊ14.6%��
��3��ǡ����ȫ��Ӧʱ���ʵ���������Ϊ20%��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
һ��ˮ�տ����DZ�������ԭ���ǣ�������
| A��ˮ���ӱ���� |
| B��ˮ�ֽ�������������� |
| C���������ʵ�������Ŀ���� |
| D��ˮ��Һ̬��Ϊ��̬�����Ӽ�ļ������ |
����Mg��NO3��2��Cu��NO3��2�Ļ����Һ�У�������������۳�ַ�Ӧ����ˣ���ֽ�ϵ������ǣ�������
| A��ͭ | B���� | C��þ��ͭ | D��ͭ���� |
����Ԫ�ط�����д����ȷ���ǣ�������
| A��þmg | B��ͭCu |
| C��пZn | D����Si |
���з�Ӧ�У������кͷ�Ӧ���ǣ�������
| A��HCl+AgNO3�TAgCl��+HNO3 |
| B��3KOH+H3PO4�TK3PO4+3H2O |
| C��CuO+H2�TCu+H2O |
| D��NH4Cl+NaOH�TNaCl+NH3��+H2O |
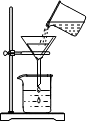 ijͬѧ�����ǵĺ�ˮ�����ձ��У��ȼ��������農�ã��ٲ�����ͼ���й��ˣ�
ijͬѧ�����ǵĺ�ˮ�����ձ��У��ȼ��������農�ã��ٲ�����ͼ���й��ˣ�