��Ŀ����
Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʡ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ�������ѧʽ��NH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽΪ��2NH3+H2SO4�T�T(NH4)2SO4��
��ȡ��ţ����Ʒ30mL�á��Ƕ�����ֽ����еĵ����ʣ������İ�����9.5g������������Ϊ4.9%��ϡ����ǡ����ȫ���ա����㲢�ش��������⣺
���ϣ���ţ��
�����ڣ�8����
��������250mL��
Ӫ���ɷ֣���ÿ100mL��
��³0.11g
֬��³3.30g
������³2.90g
��1�����������������Ƕ��ٿ�?����������ȷ��0.01g����ͬ��
��2��30mLţ���к���Ԫ�ص������Ƕ��ٿ�?
��3����ͼ�Ǹ�ţ�̰�װ��ǩ�IJ������ݡ���֪ţ���еĵ����ʺ���Ԫ�ط���Ϊ16%������ͨ������ȷ������ţ����Ʒ�е����ʵĺ����Ƿ�ﵽ�˰�װ����ʾ�ĵ����ʵ���������
�𰸣�
������
��ʾ��
������
| ��1��0.16g�� ��2��0.13g
��3��2.7<2.90û�д�ꡣ
|
��ʾ��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
 Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʣ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ�������ѧʽΪ��NH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽ��
Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʣ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ�������ѧʽΪ��NH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽ��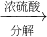 �����
�����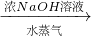 ����Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ�����ȡ30mLţ���øǶ�����뵰���ʣ��ѵ�Ԫ����ȫת���ɰ���NH3������50g4.9%ϡH2SO4��Һ���պ�ʣ�������Ҫ��38.0g4.0%��NaOH��Һ�кͣ���
����Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ�����ȡ30mLţ���øǶ�����뵰���ʣ��ѵ�Ԫ����ȫת���ɰ���NH3������50g4.9%ϡH2SO4��Һ���պ�ʣ�������Ҫ��38.0g4.0%��NaOH��Һ�кͣ���