��Ŀ����
��6�֣�ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����鿴�����ѹ��ڣ�����֪�Ƿ���ȫ���ʣ�����������ƿ˫��ˮ����ѧУ����������ͼ9��ʾ��̽�����Իش�
(1)�����ƿ˫��ˮδ��ȫ���ʣ��ɹ۲쵽�Թ��������ݲ������Ҵ����ǵ�ľ��_______________�������ܹ۲쵽��������˵����ƿ˫��ˮ�Ѿ���ȫ���ʱ��_________��
(2) �Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ�� ��
����������̵�Ŀ����__________________��
(3)����δ���ڵ�������˫��ˮ�����˿��ϣ���Ѹ�ٳ��ִ������ݣ�����Ϊ�����������
_______��
|

���𰸡�
��6�֣��Ÿ�ȼ ˮ ��ÿ�ո�1�֣�
�� 2H2O2 MnO2======2H2O+ O2����2�֣� ������ ��1�֣�
������ ��1�֣�
����������

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ


 ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����鿴�����ѹ��ڣ�����֪�Ƿ���ȫ���ʣ�����������ƿ˫��ˮ����ѧУ����������ͼ��ʾ��̽�����Իش�
ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����鿴�����ѹ��ڣ�����֪�Ƿ���ȫ���ʣ�����������ƿ˫��ˮ����ѧУ����������ͼ��ʾ��̽�����Իش� 30��ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�
30��ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�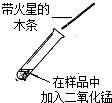 ��2009?Ӫ��ģ�⣩ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�������ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�
��2009?Ӫ��ģ�⣩ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�������ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�

