��Ŀ����
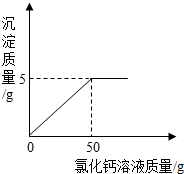
 �ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺
�ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺��1��������Ʒ��NaOH��������
��2����������CaCl2��Һ����������������
��3��ǡ����ȫ��Ӧʱ�ձ�����Һ��pH
��
��
7��ѡ���������������=��������������1�����ʵ��������ƺ���̼���ƣ����Ȼ��Ʒ�Ӧ������̼��Ƴ�������ͼ��֪����50g�Ȼ�����Һ̼����������ȫ��Ӧ������̼���������5g���Ӷ������������Ƶ�������
��2������̼��Ƶ����������Ȼ��Ƶ���������������Ȼ��Ƶ�����������
��3��ǡ�÷�Ӧʱ�������ƴ�������Һ�У���Һ�ʼ��ԣ�
��2������̼��Ƶ����������Ȼ��Ƶ���������������Ȼ��Ƶ�����������
��3��ǡ�÷�Ӧʱ�������ƴ�������Һ�У���Һ�ʼ��ԣ�
����⣺���ʵ��������ƹ����г�����̼���ƣ�̼�������Ȼ��Ʒ�Ӧ������̼��Ƴ�������ͼ��֪����50g�Ȼ�����Һ̼����������ȫ��Ӧ������̼���������5g���Ӷ������������Ƶ�����
����Ʒ��̼���Ƶ�����Ϊx��������Ȼ�����Һ�е���������Ϊy
Na2C03+CaCl2=CaC03��+2NaCl
106 111 100
x y 5g
=
=
x=5.3g y=5.55g
����Ʒ��NaOH������Ϊl0g-5.3g=4.7g
��2������CaCl2��Һ��������������=
��l00%=11.1%
����Ʒ��NaOH������Ϊ4.7g������CaCl2��Һ��������������Ϊll��1%
��3���Ȼ�����Һ�����ԣ�����ԭ�����д����������ƹ���������Һ�ʼ���pH����7��
�ʴ�Ϊ����1����Ʒ��NaOH������Ϊ4.7g����2������CaCl2��Һ��������������Ϊ11.1%����3������
����Ʒ��̼���Ƶ�����Ϊx��������Ȼ�����Һ�е���������Ϊy
Na2C03+CaCl2=CaC03��+2NaCl
106 111 100
x y 5g
| 106 |
| x |
| 111 |
| y |
| 100 |
| 5g |
x=5.3g y=5.55g
����Ʒ��NaOH������Ϊl0g-5.3g=4.7g
��2������CaCl2��Һ��������������=
| 5.55g |
| 50g |
����Ʒ��NaOH������Ϊ4.7g������CaCl2��Һ��������������Ϊll��1%
��3���Ȼ�����Һ�����ԣ�����ԭ�����д����������ƹ���������Һ�ʼ���pH����7��
�ʴ�Ϊ����1����Ʒ��NaOH������Ϊ4.7g����2������CaCl2��Һ��������������Ϊ11.1%����3������
�����������ǶԻ�ѧ����ʽ����Ŀ��飬����Ĺؼ����ҵ���֪����������������ͼ��ķ���������ɵ�̼��Ƶ���������������������������������Ҫ�����ʵ�������ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2�TCaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺
�ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2�TCaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺ �ֳ�ȡ���ʵ�����������Ʒl0g�����Ƴ���Һ������������μ���CaCl2��Һ��CaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺
�ֳ�ȡ���ʵ�����������Ʒl0g�����Ƴ���Һ������������μ���CaCl2��Һ��CaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺ �ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺
�ֳ�ȡ���ʵ�����������Ʒ10g�����Ƴ���Һ���������м���CaCl2��Һ����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl������ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺