��Ŀ����
4����28�조�й�ˮ�ܡ�������Ϊ����Լˮ��Դ������ˮ��ȫ������1�����й���ˮ��˵���У�����ȷ���У��ڢܣ�����ţ���
��ˮ������Ԫ�غ���Ԫ����ɵĻ����� ���峺������Ȫˮ�Ǵ�����
�ۺ���ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ �ܽ�����̿����Ӳˮ�п�ʹ������
��ϴ�ˡ�ϴ�º��ˮ������������ϴ����
��2����Ȼ���е�ˮһ��Ҫ���������ʹ�ã����������ˡ���������־���ˮ�IJ����У���һ������Ծ����̶���ߵ�������
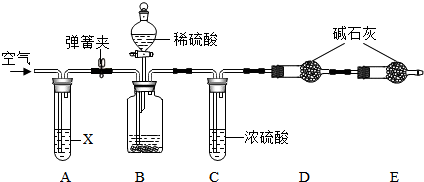
��3����ͼ��ʾ����ˮ���������з�����һ����Ӧ���۹��̣�

��д�����������е��ʵĻ�ѧʽCl2��������D����Ԫ�صĻ��ϼ�Ϊ+1�ۣ�
��4���������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ������з���������Ӳ�Ⱥ�ɱ��ԭ����������ˮ������ˮһ����ˮ���м������ˮ��
���� ��1���ٸ���ˮ��Ԫ����ɷ����жϣ�
�ڸ��ݴ�����Ļ�����������жϣ�
�۸���ũҩ���ʵ�ʹ�÷����жϣ�
�ܸ���Ӳˮ�����ķ��������жϣ�
�ݸ��ݽ�Լ��ˮ�ķ����жϣ�
��2����������������ֻ�ܳ�ȥˮ�еIJ��������ʣ����ܳ�ȥˮ�п����Ե����ʣ�������Գ�ȥˮ�п����Ե����ʵõ���ˮ������
��3���ٸ���B���ӵĹ��ɿ�֪B�Ļ�ѧʽ��
�ڸ��ݻ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�������㣮
��4������Ӳˮ����ˮ�ķ���������Ӳˮ����ˮ�ķ����ǣ��÷���ˮ���������ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ������ˮ��Ӳ�ȵķ����ǣ�������С�����
��� �⣺��1���ٸ���ˮ�Ļ�ѧʽ��֪ˮ������Ԫ�غ���Ԫ����ɵĻ������˵����ȷ��
��Ȫˮ�ܽ��п����Ե����ʣ���������˵������ȷ��
�ۺ���ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ����˵����ȷ��
�ܻ���̿����Ӳˮ������ֻ̿��������ζ��ɫ�أ��������ո�þ���ӣ����Ի���̿��������Ӳˮ����˵������ȷ��
��ϴ�ˡ�ϴ�º��ˮ������������ϴ������һˮ���ã��ɽ�Լ��ˮ����˵����ȷ��
�ʴ�ѡ�ڢܣ�
��2������������ֻ�ܳ�ȥˮ�еIJ��������ʣ����ܳ�ȥˮ�п����Ե����ʣ�������Գ�ȥˮ�п����Ե����ʵõ���ˮ���ʴ𰸣�����
��3����B���ӵĹ��ɿ�֪B�Ļ�ѧʽΪ��Cl2��
��D�Ļ�ѧʽΪHClO����HClO��Cl�Ļ��ϼ�Ϊx
��+1��+x+��-2��=0
���x=+1
�ʴ𰸣�+1�ۣ�
��4������Ӳˮ����ˮ�ķ����ǣ��÷���ˮ���������ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ������ˮ��Ӳ�ȵķ����ǣ�������С����ʴ�Ϊ��������У�����ˮ��
���� ������ؿ�����ˮ��Դ�ľ��������úͱ������ֽ��ˮ����ɽ��п��飬�ۺ��Խ�ǿ��

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�| A�� | ������������ | B�� | ��������Ԫ�� | C�� | ��������ԭ�� | D�� | ���ǵ��ʷ��� |
| A�� | K2CO3 Al��OH��3 | B�� | CaOH Fe2��SO4��3 | C�� | Fe FeCl | D�� | NH4Cl2 NH3 |

| A�� | �ô˷�������װ��������������γ� | |
| B�� | �˹�����û�зֽⷴӦ | |
| C�� | �˹�����SԪ�صĻ��ϼ�δ�����ı� | |
| D�� | �������̵ķ�Ӧ�ɱ�ʾΪ��2SO2+2CaCO3+O2�T2CaSO4+2CO2 |
| A�� | ���о�һ�ԡ��ȶ��Ե�Һ��һ������Һ | |
| B�� | ������Һʱ�������������������ʵ��ܽ�� | |
| C�� | ������Һ���º�һ������������ | |
| D�� | �¶Ȳ���ʱ��KCl�ı�����Һ��������������һ������ |
| A�� | ��ʯ��ˮ�Լ������б��� | |
| B�� | ��ʳ��ˮ��ȥˮ�����ˮ�� | |
| C�� | ʳƷ������뵪���ӳ�ʳƷ�ı����� | |
| D�� | �ϳ涣ҧ�����˵�Ƥ���ڷ��ڳ����ᣬ��ͿһЩ��NH3•H2O��ҩˮ����ʹ�� |
 ���ݵĴ��С��ֲ��д���ͭ�Ƴ��е������ѳ�����ɫͭ�⣮ijͬѧ�������ͼʵ�飬�����ⶨ��ʽ̼��ͭ���ȷֽ�������ˮ�Ͷ�����̼�������ȣ�[��֪��ͭ�̵���Ҫ�ɷ��Ǽ�ʽ̼��ͭ�������ȷֽ�Ļ�ѧ����ʽΪCu2��OH��2CO3$\frac{\underline{\;\;��\;\;}}{\;}$2CuO+CO2��+H2O]
���ݵĴ��С��ֲ��д���ͭ�Ƴ��е������ѳ�����ɫͭ�⣮ijͬѧ�������ͼʵ�飬�����ⶨ��ʽ̼��ͭ���ȷֽ�������ˮ�Ͷ�����̼�������ȣ�[��֪��ͭ�̵���Ҫ�ɷ��Ǽ�ʽ̼��ͭ�������ȷֽ�Ļ�ѧ����ʽΪCu2��OH��2CO3$\frac{\underline{\;\;��\;\;}}{\;}$2CuO+CO2��+H2O]ʵ���õ�����Ҫװ����ͼ��ʾ��
��1��װ������˳��Ϊ���ڢ٢ۣ���д��ţ���
��2���������輰���ݴ�����
��һ�� ��������ԣ���д�������з���װ�õ������Եľ��巽���ڵ��������Ӵ��н��ܵĵ��ܣ��Ȱѵ��ܿ�����ʢ��ˮ��ˮ���У������Թܣ��۲쵼�ܿ���������ð��������֤��װ�ò�©����
�ڶ��� װ��ҩƷ����ȡװ�âٺ͢۵�������
������ ������ٴγ�ȡװ�âٺ͢۵���������¼���������
| ʵ��ʱ�� | װ�â����� | װ�â����� |
| ʵ��ǰ | 220.0�� | 195.0�� |
| ʵ��� | 222.1�� | 199.4�� |
��3�����������������ѡ����ѡ��������ʵ�������ܵ�ԭ������Щ��AC
A��������̼û�б���ȫ���� B��ˮû�б���ȫ����
C����ʽ̼��ͭҩƷ�к���ˮ�� D����ʽ̼��ͭû����ȫ�֣�