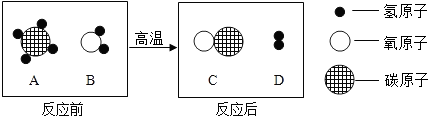
��Ŀ����
��ʵġ�̼�������˵����������һ���߽���̼��������
��1�����ݱ�1�ṩ����Ϣ����д�йغ�̼���ʵĶ�Ӧ���ԡ�
��1�����ݱ�1�ṩ����Ϣ����д�йغ�̼���ʵĶ�Ӧ���ԡ�

��2��Һ̬������̼�� �����������˾ȵ��������ҷ����Ļ��֣�����˵����ȷ���� �����ţ���
�����������˾ȵ��������ҷ����Ļ��֣�����˵����ȷ���� �����ţ���
A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3������Ķ�����̼�Ӿ��ˡ�����ЧӦ����д��һ�����ٶ�����̼�ŷŵĽ��� ��
��4��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO ��NH2��2] ��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú�� ����Ȼ�������Ƕ����� �������������������������Դ��
��6����Ȼ���м�����ȫȼ�յĻ�ѧ����ʽΪ
��7���ӱ�2���ݷ�������ú��ȣ�����Ȼ����ȼ�ϵ��ŵ��� ��
 �����������˾ȵ��������ҷ����Ļ��֣�����˵����ȷ���� �����ţ���
�����������˾ȵ��������ҷ����Ļ��֣�����˵����ȷ���� �����ţ��� A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3������Ķ�����̼�Ӿ��ˡ�����ЧӦ����д��һ�����ٶ�����̼�ŷŵĽ��� ��
��4��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO ��NH2��2] ��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú�� ����Ȼ�������Ƕ����� �������������������������Դ��
��6����Ȼ���м�����ȫȼ�յĻ�ѧ����ʽΪ
��7���ӱ�2���ݷ�������ú��ȣ�����Ȼ����ȼ�ϵ��ŵ��� ��

�Ţ�Ӳ�ȴ� �ڵ����� ��������
��AB
�ǽ�Լ��ֽ���������ɣ�
�� CO2+2NH3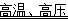 CO��NH2��2+H2O
CO��NH2��2+H2O
��ʯ�͡���������
��CH4+2O2 CO2+2H2O
CO2+2H2O
�˵���������Ȼ����ú��ȫȼ�գ���Ȼ��ȼ�ղ���������̼������С��ú���ų�����������ú��
��AB
�ǽ�Լ��ֽ���������ɣ�
�� CO2+2NH3
 CO��NH2��2+H2O
CO��NH2��2+H2O ��ʯ�͡���������
��CH4+2O2
 CO2+2H2O
CO2+2H2O�˵���������Ȼ����ú��ȫȼ�գ���Ȼ��ȼ�ղ���������̼������С��ú���ų�����������ú��

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
 ��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮
��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮