��Ŀ����
 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
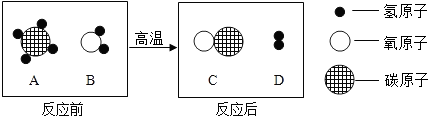
��ʵġ�̼�������˵����������һ���߽���̼�������磮��1�����ݱ����ṩ����Ϣ����д�йغ�̼���ʵĶ�Ӧ���ԣ�
| ������; | һ����̼��ȼ�� | ʯī���缫 | ����̿��ˮ |
| ��Ӧ���� | �� ��ȼ�� ��ȼ�� |
�� ������ ������ |
�� ������ ������ |
AB
AB
������ĸ��ţ���A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3���� 440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ
4Na+CO2
2Na2CO3+C
| ||
| ��ѹ |
4Na+CO2
2Na2CO3+C
��
| ||
| ��ѹ |
��4����̼���ࡱ����֪����Ĺ�����ϣ���ͼ��ʾ������̼Ԫ����ɣ����ж�ṹ�����Ժã�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵����ȷ����
ABC
ABC
������ĸ��ţ���A������������ B�����ظ�ʹ�� C���ɴ�������ʯ��й©��
��������1������һ����̼���п�ȼ�ԡ�ʯī���е����ԡ�����̿���������Խ��н��
��2�����ݶ�����̼����ԭ�����н��
��3��������440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƽ��н��
��4��������Ŀ����Ϣ��֪̼��������ʺ���;���н��
��2�����ݶ�����̼����ԭ�����н��
��3��������440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƽ��н��
��4��������Ŀ����Ϣ��֪̼��������ʺ���;���н��
����⣺��1��һ����̼���п�ȼ�ԣ�������ȼ�ϣ�ʯī���е����ԣ��������缫������̿���������ԣ������ھ�ˮ�������ȼ�ԣ������ԣ������ԣ�
��2��A��Һ̬������̼��������Ⱦ�������ϣ���A��ȷ��
B��������̼�ܶȱȿ����ɸ�����ȼ������棬�����������Ӷ����������ã���B��ȷ��
C��Һ̬������̼����ʱ���ȣ����ǿ�ȼ����Ż���Dz���ģ����ܽ��ͣ���C����
��ѡ��AB��
��3����440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ4Na+CO2
2Na2CO3+C�����4Na+CO2
2Na2CO3+C��
��4��A����̼���ࡱ���ж�ṹ�����Ժã�����ʯ���к�ǿ���������������Ծ��������ԣ���A��ȷ��
B���������ʯ�ͼ������Կɻָ�ԭ״�����Կ��ظ�ʹ�ã���B��ȷ��
C��̼�����ʯ���к�ǿ���������������Կɴ�������ʯ��й©����C��ȷ��
��ѡ��ABC��
��2��A��Һ̬������̼��������Ⱦ�������ϣ���A��ȷ��
B��������̼�ܶȱȿ����ɸ�����ȼ������棬�����������Ӷ����������ã���B��ȷ��
C��Һ̬������̼����ʱ���ȣ����ǿ�ȼ����Ż���Dz���ģ����ܽ��ͣ���C����
��ѡ��AB��
��3����440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ4Na+CO2
| ||
| ��ѹ |
| ||
| ��ѹ |
��4��A����̼���ࡱ���ж�ṹ�����Ժã�����ʯ���к�ǿ���������������Ծ��������ԣ���A��ȷ��
B���������ʯ�ͼ������Կɻָ�ԭ״�����Կ��ظ�ʹ�ã���B��ȷ��
C��̼�����ʯ���к�ǿ���������������Կɴ�������ʯ��й©����C��ȷ��
��ѡ��ABC��
������������Ҫ����������ЧӦ�����⣮���������ǿ����г����ֵ����ſ��㣬�˽���ֻ�������IJ����Լ���Ӧ�ķ��δ�ʩ�ǽ���������Ĺؼ���

��ϰ��ϵ�д�
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
 ��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮
��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮