��Ŀ����
����ơ���һ�ֿ�����ϸ �����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȣ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���
�����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȣ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���
��1��̼����и�Ԫ�ص�����������_________%��ʳ��������̼��������ڷ�ֹ����ȱ�������_________�����������֢����ƶѪ��������
��2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����_________���������Ӧ���ͣ���
��3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽_________��֤�������к���ʯ��ʯ��
��4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ��������ƣ�һ����Ҫ�ļ����Ӧ��ԭ����_________����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�
��40����������֢
��2���ֽⷴӦ
��3�������ݲ���
��4��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��2�֣�
�⣺��õ��������Ƶ�����Ϊ x�� ��1�֣�
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
100 80 ��1�֣�
50t x ��1�֣�
 =
= . ��1�֣�
. ��1�֣�
x=40t ��1�֣�
���ܵõ��������Ƶ�����Ϊ40t�� ��1�֣�

ʹ����ͼ��ʾװ����ȡ������

��1��д�����ȸ�������������Ļ�ѧ����ʽ�� ��
��2��ָ��ͼ4װ����1�����ԵIJ���֮���� ��
��3�������ռ���ɺ���䴿������ƫ�ͣ�ԭ������ǣ� ������ĸ���ɶ�ѡ����
A����������л����˶�������
B���ռ�ǰ������ƿ��δע��ˮ
C���ռ�����ƿ����������ˮ
D��δ��������������ð��ʱӦ��ʼ�ռ�
��4�����о���������ȡ���������ʼ��䷴Ӧ�������Ĺ����У�ijͬѧ����������ʾ��ϵ��ʵ�飺
| ��� | ʵ�� | �ܷ��������� |
| A | ���ȸ������ | �� |
| B | ���ȶ������� | ���� |
| C | ���������� | ���� |
| D | ����ˮ | ���� |
| E | ���ˮ | �� |
�ٸ���ʵ��A��B��C�����Եó��Ľ����ǣ���һ�������£� ��
�ڸ���ʵ��D��E�����Եó��Ľ����ǣ� ��Ӱ�������ܷ�Ӧ������������Ҫ���أ�
��һ���ܱ������з���X��Y��Z��W�������ʣ���һ�������·�����ѧ��Ӧ��һ��ʱ�����й��������±�������ڴ˷�Ӧ����ʶ��ȷ���ǣ�������
| ���� | X | Y | Z | W |
| ��Ӧǰ��������g�� | 2 | 1 | 16 | 16 |
| ��Ӧ���������g�� | 17 | m | 6 | 11 |
A���÷�Ӧ�û�ѧ����ʽ��ʾΪ��3X+Y=2Z+W
B���÷�Ӧ�Ļ�������Ϊ���Ϸ�Ӧ
C��m=2
D����Ӧ��������Z��Y��������Ϊ1��6
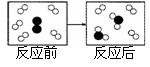


 A����Ȼ��������Ԫ�ص�ԭ�Ӷ������Ӻ����ӹ���
A����Ȼ��������Ԫ�ص�ԭ�Ӷ������Ӻ����ӹ���

 Ϊ�˵õ������������CO2������װ�ã�����ͼ��ʾ���ĵ���
Ϊ�˵õ������������CO2������װ�ã�����ͼ��ʾ���ĵ���
 �IJ�������Һ�в��ϼ�����������
�IJ�������Һ�в��ϼ�����������