��Ŀ����
 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�������1�����������ϣ���֪��ʽ̼��ͭ�����������ʣ�����Ϊ�������ڻ�ѧ���ʵ���
��2�����ȿ�ȸʯ��Ʒʹ֮�ֽ⣬�䷴Ӧ�Ļ�ѧ����ʽ��
��3���������ɵ��������Ƿ���CO2 ��Ҫ�õ��Ļ�ѧ�Լ�������
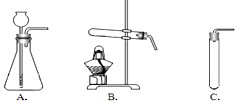
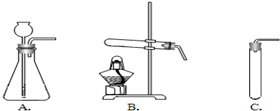
����������̽����ʽ̼��ͭ[Cu2��OH��2CO3]���й����ʣ���Ҫ����ֽ�Ļ�ѧ���ʣ���ʽ̼��ͭ[Cu2��OH��2CO3]Ϊ���壬��Ҫ���Ȳ��ܷ�Ӧ����װ��Ҫѡ����ȵģ��ó���ʯ��ˮ�����Ƿ���CO2����ľ̿��ԭ����ͭ�������û���Ӧ�����ݷ�Ӧ��������������غ㶨����д��ѧ����ʽ��
����⣺��1�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ���ڻ�ѧ���ʣ��ʴ�Ϊ��D
��2����ʽ̼��ͭ[Cu2��OH��2CO3]Ϊ���壬��Ҫ���Ȳ��ܷ�Ӧ�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ���������غ��д����ѧ����ʽ���ʴ�Ϊ��Cu2��OH��2CO3
2CuO+CO2��+H2O����B����Ӧ��Ҫ���ȣ�C+2CuO
2Cu+CO2��
��3���ó���ʯ��ˮ�����Ƿ���CO2������ʯ��ˮ����Ǿ�֤��ʵ������CO2������������̼��ʯ��ˮ�е��������Ʒ�Ӧ����̼��Ƴ�����ˮ�����������غ��д����ѧ����ʽ���ʴ�Ϊ������ʯ��ˮ������ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O
��2����ʽ̼��ͭ[Cu2��OH��2CO3]Ϊ���壬��Ҫ���Ȳ��ܷ�Ӧ�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ���������غ��д����ѧ����ʽ���ʴ�Ϊ��Cu2��OH��2CO3
| ||
| ||
��3���ó���ʯ��ˮ�����Ƿ���CO2������ʯ��ˮ����Ǿ�֤��ʵ������CO2������������̼��ʯ��ˮ�е��������Ʒ�Ӧ����̼��Ƴ�����ˮ�����������غ��д����ѧ����ʽ���ʴ�Ϊ������ʯ��ˮ������ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O
����������Ϊ̽���⣬̽����ȸʯ�ijɷ��Լ��й����ʣ�ʵ��̽����ľ̿��ԭ����ͭ������ɣ�

��ϰ��ϵ�д�
�����Ŀ


 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����