��Ŀ����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2(OH)2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
(1)���������ϣ���֪��ʽ̼��ͭ�����������ʣ�����Ϊ�������ڻ�ѧ���ʵ���__________��
A����ɫ����B��������ˮC�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ
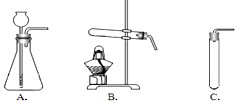
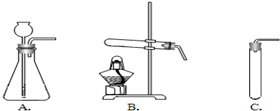
(2)���ȿ�ȸʯ��Ʒʹ֮�ֽ⣬�䷴Ӧ�Ļ�ѧ����ʽ��___________________________Ӧѡ������_________(��װ�õ����)װ�ã���������____________________________________��
(1)���������ϣ���֪��ʽ̼��ͭ�����������ʣ�����Ϊ�������ڻ�ѧ���ʵ���__________��
A����ɫ����B��������ˮC�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ
(2)���ȿ�ȸʯ��Ʒʹ֮�ֽ⣬�䷴Ӧ�Ļ�ѧ����ʽ��___________________________Ӧѡ������_________(��װ�õ����)װ�ã���������____________________________________��
��ȤС����ݳ��пα���ľ̿��ԭ����ͭ��ԭ����һ��ʵ��õ���ɫ����ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________ ��
(3)�������ɵ��������Ƿ���CO2 ��Ҫ�õ��Ļ�ѧ�Լ�������________________���۲쵽___________ʱ��֤��ʵ������CO2�������÷�Ӧ�Ļ�ѧ����ʽ��_________________________ ��
(3)�������ɵ��������Ƿ���CO2 ��Ҫ�õ��Ļ�ѧ�Լ�������________________���۲쵽___________ʱ��֤��ʵ������CO2�������÷�Ӧ�Ļ�ѧ����ʽ��_________________________ ��
(1) C
(2) Cu2(OH)2CO3 2CuO+H2O+CO2����B�������Ƿ�Ӧ��Ҫ���ȣ�C+2CuO
2CuO+H2O+CO2����B�������Ƿ�Ӧ��Ҫ���ȣ�C+2CuO 2Cu+CO2��
2Cu+CO2��
(3)����ʯ��ˮ������ʯ��ˮ����ǣ�CO2+Ca(OH)2==CaCO3��+H2O
(2) Cu2(OH)2CO3
 2CuO+H2O+CO2����B�������Ƿ�Ӧ��Ҫ���ȣ�C+2CuO
2CuO+H2O+CO2����B�������Ƿ�Ӧ��Ҫ���ȣ�C+2CuO 2Cu+CO2��
2Cu+CO2��(3)����ʯ��ˮ������ʯ��ˮ����ǣ�CO2+Ca(OH)2==CaCO3��+H2O

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ


 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽����� ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����