��Ŀ����
10�� �����dz��õ����ʼ��鷽��֮һ���Ǹ���һ�����ʵ��������ʣ��û�ѧ�����������Dz����������ʣ�
�����dz��õ����ʼ��鷽��֮һ���Ǹ���һ�����ʵ��������ʣ��û�ѧ�����������Dz����������ʣ���1���ټ�ͬѧѧϰũ������������������ʯ�ҡ�Ca��OH��2��������立���������꣬ͨ������ζ�������Dz��ǵ��ʣ����û�ѧ����ʽ��ʾ��������ԭ������NH4��2SO4+Ca��OH��2=CaSO4+2NH3��+2H2O��
����ͬѧ�������ͼ��ʾ�ķ���������Σ���������李��������ơ���ֽ��δʧЧ����һ��ʱ�������ֽ����ɫ�����Ա仯�����ܵ�ԭ���ǣ�û�м��ȣ���Ӧ��Ũ�ȹ��ͣ���д��һ��ԭ�ɣ�
��2����ij��Һ�к��нϴ�����Na+��Cl-��CO${\;}_{3}^{2-}$��SO${\;}_{4}^{2-}$�������ӣ���ʦҪ��ͬѧ�Ƿֱ��������Լ��Ⱥ��������������ӣ�Ҫ��ÿ����һ���Լ��ܹ������һ�����ӣ������Ҵ�����Һֻ��ȡһ�Σ���һ��������ȡһ�Թܣ����������Һ�������������м��������ʵ������һ�������ǣ����ã����ϲ���Һ�м�������������Һ��ȷ����������ӵĴ��ڣ�
���� ��1���������������ʯ�ҵķ�Ӧд����Ӧ�Ļ�ѧ����ʽ������֮�䷴Ӧ���������¶ȡ�Ũ�ȵȷ���������йأ�
��2�����������ӿ���ʹ�������ӣ�������������ӿ���ʹ�ñ����ӣ�����̼������ӿ���ʹ�������ӣ�
��� �⣺��1���������������ʯ�ҷ�Ӧ�ų���������Ӧ�ķ���ʽ�ǣ���NH4��2SO4+Ca��OH��2=CaSO4+2NH3��+2H2O�������NH4��2SO4+Ca��OH��2=CaSO4+2NH3��+2H2O��
���������Һ������������Һ��Ũ�Ⱥ�С����Ϻ�û�м��ȣ���Ӧ���ʺ��������ɵİ������٣�����ʹʪ��ĺ�ɫʯ����ֽ���������û�м��ȣ���Ӧ��Ũ�ȹ��ͣ�
��2���������Լ����������Ӽ��������ÿ��һ���Լ��ܹ������һ�����ӣ���ʱҪע��ÿ����һ���Լ�ֻ����һ�����ӽ�ϣ����������ӿ���ʹ�������ӣ�������������ӿ���ʹ�ñ����ӣ�����̼������ӿ���ʹ�������ӣ��������Ӻͱ����Ӷ�������̼������ӽ�ϳɳ������������ȼ���̼������ӣ���ʱ���Լ���ϡ���ᣬ�������������õ������ӿ�������������ӽ�ϳ�����ˮ�����������������ȼ��鱵���ӣ���ʱ���Լ������ᱵ�������������ӣ����Լ��������������Ա����Ϊ�������������
���� �⿼���˳������ӵļ��飬��ɴ��⣬�����������е�֪ʶ���У�ͬʱ�迼������֮��ķ�Ӧ��

 ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飺ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ʵ�����ݼ�¼���£�
ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飺ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ʵ�����ݼ�¼���£�| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ÿ�μ�����ǰ�ձ�����ʢ ���ʵ�������/g | 158.0 | 181.2 | X | 228.6 |
| �����������Һ����/g | 25.0 | 25.0 | 25.0 | 25.0 |
| ��ַ�Ӧ���ձ�����ʢ����������/g | 181.2 | 204.4 | 228.6 | 253.6 |
| ÿ�����ɵĶ�����̼����/g | 1.8 | 1.8 | Y | 0 |
��2������ͨ������˵���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%
��3������ʵ�����ݣ�ijͬѧ����ͼ����ֽ�ϻ��Ƴ�������ϡ������������������������ϵ�������������ͼ��ת�۴�P������꣮������һλС����
�±��������г���������Һ��pH����Ҫ�ɷֻ�ѧʽ��
| ��� | �� | �� | �� | �� | �� |
| �� �� | ʳ �� | �� �� | ����ˮ | ʯ��ˮ | ������Һ |
| ��Ҫ�ɷ� | CH3COOH | C2H5OH | C12H22O11 | Ca��OH��2 | Na2CO3 |
| ��ҺpH | 3 | 7 | 7 | 11 | 8 |
���������У�������ǿ���Ǣܣ�����ţ��������Ʒ䶣ҧ���ͷ�һ�ּ������ʣ���Ϊ����֢״�����ڶ�ҧ��Ϳ�٣�����ţ�
| A�� | ��������ȼ�� | B�� | ʳ��ĸ��� | C�� | ������ | D�� | �������� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | 25% | B�� | 20% | C�� | 12.8% | D�� | 6.4% |
| A�� | NaOH��Һ�л���Ba��OH��2��K2SO4�� | B�� | CO2�л���HCl���壨NaOH��Һ�� | ||
| C�� | CO2�л���CO�����ȵ�̿�� | D�� | Cu��NO3��2��Һ�л���AgNO3��Cu�ۣ� |
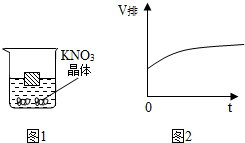 ��ͼ1��һľ��Ư����50�������ر�����Һ�У����¶ȸı�ʱ���������ɴ������ľ�����Һ����ı仯����ľ���ſ�Һ��������V������ʱ�䣨t��������ͼ2��ʾ�ı仯���ɴ��Ƴ��¶ȵĸı䷽ʽΪ���£�����¡����¡��������¶ȵĸı䣬�������Һ�����ʵ��������������٣�����䡱���������١�����
��ͼ1��һľ��Ư����50�������ر�����Һ�У����¶ȸı�ʱ���������ɴ������ľ�����Һ����ı仯����ľ���ſ�Һ��������V������ʱ�䣨t��������ͼ2��ʾ�ı仯���ɴ��Ƴ��¶ȵĸı䷽ʽΪ���£�����¡����¡��������¶ȵĸı䣬�������Һ�����ʵ��������������٣�����䡱���������١�����