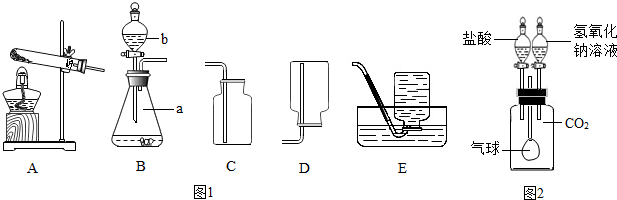
��Ŀ����
�������Ĵ����Ʒ�������Ậ���������Ȼ��ƣ�Ϊ�˲ⶨ����ɣ�ijУ��ѧ��ȤС���ͬѧ�������������ʵ�飺
��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�����۲쵽 ����֤������Ʒ�к����Ȼ��ƣ�
��2��Ϊ�ⶨ�ò�Ʒ��̼���Ƶĺ�����������ͼ����ʵ�飺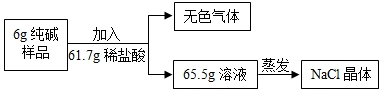
�ٸ��������غ㶨�ɣ���ʵ��������ɫ����������� g��
�ڼ����6g ������Ʒ��̼���Ƶ���������д��������̣�����������ȷ��0.1g��
�����������þ����������м��㣬��������� �����ƫ����ƫС������Ӱ�족��
��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�����۲쵽
��2��Ϊ�ⶨ�ò�Ʒ��̼���Ƶĺ�����������ͼ����ʵ�飺

�ٸ��������غ㶨�ɣ���ʵ��������ɫ�����������
�ڼ����6g ������Ʒ��̼���Ƶ���������д��������̣�����������ȷ��0.1g��
�����������þ����������м��㣬���������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,֤������Ϳ�����������
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1�����������ӵļ����������ǣ�
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼��������
�ڸ��ݻ�ѧ����ʽ���ö�����̼����������̼���Ƶ�������
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼��������
�ڸ��ݻ�ѧ����ʽ���ö�����̼����������̼���Ƶ�������
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
����⣺��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�������ְ�ɫ��������֤������Ʒ�к��������ӣ����ڸ��⣬�������Ȼ��ƣ�
�ʴ�Ϊ�����ְ�ɫ������
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼������������6g+61.7g-65.5g=2.2g��
�ʴ�Ϊ��2.2g��
�ڽ⣺��Na2CO3����Ϊx����
Na2CO3+2HCl=2NaCl+H2O+CO2�� ��1�֣�
106 44
x 2.2g
=
��� x=5.3g
�𣺸���Ʒ�к���5.3g Na2CO3��
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
�ʴ�Ϊ��ƫ��
�ʴ�Ϊ�����ְ�ɫ������
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼������������6g+61.7g-65.5g=2.2g��
�ʴ�Ϊ��2.2g��
�ڽ⣺��Na2CO3����Ϊx����
Na2CO3+2HCl=2NaCl+H2O+CO2�� ��1�֣�
106 44
x 2.2g
| 106 |
| 44 |
| x |
| 2.2g |
��� x=5.3g
�𣺸���Ʒ�к���5.3g Na2CO3��
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
�ʴ�Ϊ��ƫ��
������������Ҫ���������ӵļ��������Լ����ݻ�ѧ����ʽ�ļ��㣬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
��ʡú��ȫ�������Ʋ����ֹۣ����±�ը�¹ʵ���ҪΣ�������ǣ�������
| A������ | B��һ����̼ |
| C������ | D��������̼ |
���������У���Ԫ�صĻ��ϼ���ߵ��ǣ�������
| A��Na2SO4 |
| B��SO2 |
| C��S |
| D��H2S |
�����й���ϵͳĿǰ�Ѿ��ƹ�ʹ�ý��ܼ��ŵ�˫ȼ�Ϲ���������ͼΪ������ȼ����Ҫ�ɷ���ȫȼ�յĻ�ѧ��Ӧ����ʾ��ͼ��������˵������ȷ���ǣ�������
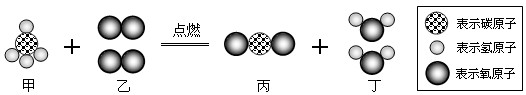

| A���÷�Ӧ�з��ӡ�ԭ�ӵ���������˸ı� |
| B�����ʼĻ�ѧʽ��CH4 |
| C���������е�Ԫ�ػ��ϼ۳�-2�� |
| D��ͼʾ��Ӧ���ڻ��Ϸ�Ӧ |