��Ŀ����
7��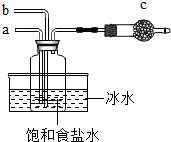 ��Ȼ����������̼�����������壬�ֶ��ǻ���ԭ�ϣ�
��Ȼ����������̼�����������壬�ֶ��ǻ���ԭ�ϣ���1����ͼ��ģ�⡰�����Ƽ����ȡNaHCO3�IJ���װ�ã�
��ѧ��Ӧԭ����NaCl+CO2+X+H2O=NaHCO3��+NH4Cl
�������̣���ͨ������X���壬��ͨ�������̼��
X�Ļ�ѧʽΪNH3���ӹ��ƿ�з����̼�����ƾ���IJ��������ǹ��ˣ�
��2�������ǻ���������Ҫ��Դ������C8H18��ʾ���͵ijɷ֣�����ʹ�����ͻᵼ������ЧӦ�Ӿ磬д��������ȫȼ�յĻ�ѧ����ʽ2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$16CO2+18H2O��
��3���ڴ��������������£�������̼��������һ��������Ӧ��������Դ�����ѣ�CH3OCH3����ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ2CO2+6H2$\frac{\underline{MnO_2}}{��}$CH3OCH3+2H2O��
���� ��1�����������غ㶨�ɿ�֪���ڻ�ѧ��Ӧǰ��ԭ�Ӹ������䣬���˿��Խ������Һ�������з�����
��2�����ݶ�����̼�ŷŻ�Ӿ�����ЧӦ�����ͺ������ڵ�ȼ������������ˮ�Ͷ�����̼���з�����
��3�������ڴ��������������£�������̼��������һ��������Ӧ��������Դ�����ѣ�CH3OCH3����ˮ���з�����
��� �⣺��1�����������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��ԭ�Ӹ������䣬����X��NH3���ӹ��ƿ�з����̼�����ƾ���IJ��������ǹ��ˣ�
��2��������̼�ŷŻ�Ӿ�����ЧӦ�����ͺ������ڵ�ȼ������������ˮ�Ͷ�����̼����ѧ����ʽΪ��2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$16CO2+18H2O��
��3���ڴ��������������£�������̼��������һ��������Ӧ��������Դ�����ѣ�CH3OCH3����ˮ����ѧ����ʽΪ��2CO2+6H2$\frac{\underline{MnO_2}}{��}$CH3OCH3+2H2O��
�ʴ�Ϊ����1��NH3�����ˣ�
��2������ЧӦ��2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$16CO2+18H2O��
��3��2CO2+6H2$\frac{\underline{MnO_2}}{��}$CH3OCH3+2H2O��
���� ��ѧ��Դ������������ַ�����������������ҵ�dz��л�ѧ��Ҫ��Ӧ��֮һ���ǿ����ص㣬���漰��ѧ����ʽ����д��������̼�Ͱ����������Լ��йػ�ѧ����ʽ�ļ���ȣ�

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д� ��ͼ��ʾ���������ӷ�Ӧ����X���ҷ��Ӻ�Y���ڷ��ӣ����ͼʾ��õ���Ϣ�У���ȷ���ǣ�������
��ͼ��ʾ���������ӷ�Ӧ����X���ҷ��Ӻ�Y���ڷ��ӣ����ͼʾ��õ���Ϣ�У���ȷ���ǣ�������| A�� | ���ӵ������ڻ�ѧ��Ӧ��δ�����˸ı� | |
| B�� | �÷�Ӧ�ķ�Ӧ����Ϊ�û���Ӧ | |
| C�� | ��Ӧ���ɵ����ʱ����ڵ��� | |
| D�� | X��Y֮�ȵ���1��3 |
 X��Y��Z�������ʵ��ܽ��������ͼ��ʾ�����������У���ȷ���ǣ�������
X��Y��Z�������ʵ��ܽ��������ͼ��ʾ�����������У���ȷ���ǣ�������| A�� | 40��ʱZ���ܽ����� | |
| B�� | �¶���60�潵��40��ʱ��Y�����ľ������ | |
| C�� | 40��ʱ��Z��Y�ı�����Һ����������������ͬ | |
| D�� | 40��ʱ��X��Y��Z�ı�����Һ������60�棬������������X=Y��Z |
| ѡ�� | ���� | ��Ҫ�ɷ� |
| A | ���� | NaHCO2 |
| B | ˮ�� | Ag |
| C | �ɱ� | H2O |
| D | ��ʯ�� | Ca��OH��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� |  �õ��� | B�� |  ���γ������� | C�� |  ��2����λ����� | D�� |  ��ѧ�����ȶ� |
 ���и������ʼ�ͨ��һ����Ӧ����ʵ����ͼת�����ǣ�������
���и������ʼ�ͨ��һ����Ӧ����ʵ����ͼת�����ǣ�������| X | Y | Z | |
| A | Fe | FeCl2 | Fe2O3 |
| B | Ca��OH��2 | NaOH | NaCl |
| C | AgNO3 | Ba��NO3��2 | BaSO4 |
| D | H2O | O2 | CuO |
| A�� | A | B�� | B | C�� | C | D�� | D |
 A��B��C��D��E��Ϊ���л�ѧ�в�ͬ�������ʣ�A��C������ͬԪ�أ�E����Ԫ�أ����ǵı仯��ϵ��ͼ��ʾ�����к�ɫ����A��һ�������¿���ת��Ϊ����B����-����ʾ���������������ܷ�����Ӧ����
A��B��C��D��E��Ϊ���л�ѧ�в�ͬ�������ʣ�A��C������ͬԪ�أ�E����Ԫ�أ����ǵı仯��ϵ��ͼ��ʾ�����к�ɫ����A��һ�������¿���ת��Ϊ����B����-����ʾ���������������ܷ�����Ӧ����
