��Ŀ����
��ͼ��ʾΪ�������������Ʒ�Ӧ����ʾ��ͼ������ͼʾ��Ϣ�ش��������⣺��1��д��������Ӧ�Ļ�ѧ����ʽ______��
��2����֪������������Һ������Ϊ80g����������Ϊ10%������Һ�к����������Ƶ�����Ϊ______g����Ҫ��ȫ�к���Һ����Ҫ��������Ϊ20%��ϡ����______g��
��3�����ڸ÷�Ӧû�����Ե�����Ϊ�˹۲췴Ӧ�Ľ��У����������ձ�������������Һ�еμӼ��η�̪��Һ�����μ�ϡ���ᣬ��������һ���������۲쵽������______���ձ��е�pHֵ��______���������С�䡱��

���𰸡���������1�������������������Ƶķ�Ӧ��д����Ӧ�ķ���ʽ��
��2��������Һ���������������ļ��㹫ʽ���м��㣻�����������������Ʒ�Ӧ�����������Ƶ��������������Ҫ��������Ϊ20%��ϡ�����������
��3�����ݷ�̪��Һ������ε���Һ����ɫ�ı仯������
����⣨1���������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ�ķ���ʽ�ǣ�H2SO4+2NaOH�TNa2SO4+2H2O��
��2������Һ���������������ļ��㹫ʽ��֪����Һ�к����������Ƶ�����Ϊ��80g×10%=8g��
����Ҫ��������Ϊ20%��ϡ���������ΪX
H2SO4+2NaOH�TNa2SO4+2H2O
98 80
X×20% 8g
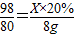 ��ã�X=49g
��ã�X=49g
��3�������������Ƴʼ��ԣ���ʹ��̪��Һ���ɫ�����������������Ʒ����кͷ�Ӧ�����������ƺ�ˮ�������Ƴ����ԣ�����ʹ��̪��ɫ�����ԣ�
�۲쵽�������ǣ���Һ������ɫ��ɺ�ɫ�����ɺ�ɫ�����ɫ���ձ��е�pHֵ���С��
�ʴ�Ϊ����1��H2SO4+2NaOH�TNa2SO4+2H2O����2��8 g��49g����3����Һ������ɫ��ɺ�ɫ�����ɺ�ɫ��Ϊ��ɫ����С��
������������Ҫ�������кͷ�Ӧ��֪ʶ��������кͷ�Ӧ������Һ������Եı仯�Լ�ָʾ���ı仯�ȣ�
��2��������Һ���������������ļ��㹫ʽ���м��㣻�����������������Ʒ�Ӧ�����������Ƶ��������������Ҫ��������Ϊ20%��ϡ�����������
��3�����ݷ�̪��Һ������ε���Һ����ɫ�ı仯������
����⣨1���������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ�ķ���ʽ�ǣ�H2SO4+2NaOH�TNa2SO4+2H2O��
��2������Һ���������������ļ��㹫ʽ��֪����Һ�к����������Ƶ�����Ϊ��80g×10%=8g��
����Ҫ��������Ϊ20%��ϡ���������ΪX
H2SO4+2NaOH�TNa2SO4+2H2O
98 80
X×20% 8g
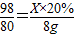 ��ã�X=49g
��ã�X=49g��3�������������Ƴʼ��ԣ���ʹ��̪��Һ���ɫ�����������������Ʒ����кͷ�Ӧ�����������ƺ�ˮ�������Ƴ����ԣ�����ʹ��̪��ɫ�����ԣ�
�۲쵽�������ǣ���Һ������ɫ��ɺ�ɫ�����ɺ�ɫ�����ɫ���ձ��е�pHֵ���С��
�ʴ�Ϊ����1��H2SO4+2NaOH�TNa2SO4+2H2O����2��8 g��49g����3����Һ������ɫ��ɺ�ɫ�����ɺ�ɫ��Ϊ��ɫ����С��
������������Ҫ�������кͷ�Ӧ��֪ʶ��������кͷ�Ӧ������Һ������Եı仯�Լ�ָʾ���ı仯�ȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���壮

д��������������Ƣ� ��
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
��2�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣮ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����
������B�������� ��
������E������� ��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص����������� ���ƫС������ƫ������Ӱ�족��֮һ����

д��������������Ƣ�
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ���� | ����װ�� | �ռ�װ�� |
| O2 | ||
| H2 | ||
| CO2 |

������B��������
������E�������
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ
����װ����û����������B����ʹ��������������Ԫ�ص�����������
 ��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺��� ��2012?�������ģ��ijС������ͼ��ʾ��װ�öԽ���ͭ�������ɫͭ�⣨ͭ�������ijЩ���ʷ�Ӧ�IJ�������о���ʵ���й۲쵽�������ǣ�
��2012?�������ģ��ijС������ͼ��ʾ��װ�öԽ���ͭ�������ɫͭ�⣨ͭ�������ijЩ���ʷ�Ӧ�IJ�������о���ʵ���й۲쵽�������ǣ�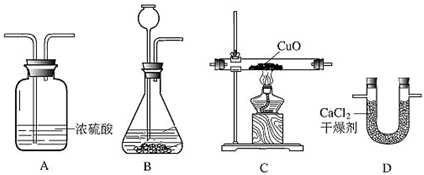
 ����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��������й���Ϣ�ش��㣺
����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��������й���Ϣ�ش��㣺