��Ŀ����
����ɫ��ѧ���ǻ�ѧ��ҵ�ķ�չ����ԭ���������Ǻ�����ɫ��ѧ����Ҫָ�꣮ԭ��������=Ŀ�IJ�������/����������×100%����ҵ��������̷����Ļ�ѧ��ӦΪ��4NH3+5O2 4NO+6H2O�� 2NO+O2=2NO2�� 3NO2+H2O=2HNO3+NO�����е�Ԫ��ȫ��ת��Ϊ������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O���ɴ˿��Ƴ�����ҵ����������������ԭ�ӵ����������ֵԼΪ�� ��
4NO+6H2O�� 2NO+O2=2NO2�� 3NO2+H2O=2HNO3+NO�����е�Ԫ��ȫ��ת��Ϊ������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O���ɴ˿��Ƴ�����ҵ����������������ԭ�ӵ����������ֵԼΪ�� ��A��52.6%
B��77.8%
C��80.7%
D��100%
���𰸡���������ҵ�������ܲ���Ϊ�����ˮ��Ŀ�IJ���Ϊ���ᣬ����ԭ��������= ×100%������
×100%������
����⣺�й�ҵ��������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O����֪��������ܲ���Ϊ�����ˮ��Ŀ�IJ���Ϊ���ᣬ
ԭ�����������ֵ= ×100%��77.8%��
×100%��77.8%��
��ѡB��
���������⿼����ɫ��ѧ����ɫ��ѧ�ֳ�ԭ�Ӿ��û�ѧ�����Ǿ����÷�Ӧ���е�ԭ�ӵ������ʴﵽ���ֵ��
 ×100%������
×100%����������⣺�й�ҵ��������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O����֪��������ܲ���Ϊ�����ˮ��Ŀ�IJ���Ϊ���ᣬ
ԭ�����������ֵ=
 ×100%��77.8%��
×100%��77.8%����ѡB��
���������⿼����ɫ��ѧ����ɫ��ѧ�ֳ�ԭ�Ӿ��û�ѧ�����Ǿ����÷�Ӧ���е�ԭ�ӵ������ʴﵽ���ֵ��

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
����ɫ��ѧ���ǻ�ѧ��ҵ�ķ�չ����ԭ���������Ǻ�����ɫ��ѧ����Ҫָ�꣮ԭ��������=Ŀ�IJ�������/������������100%����ҵ��������̷����Ļ�ѧ��ӦΪ��4NH3+5O2
4NO+6H2O�� 2NO+O2=2NO2�� 3NO2+H2O=2HNO3+NO�����е�Ԫ��ȫ��ת��Ϊ������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O���ɴ˿��Ƴ�����ҵ����������������ԭ�ӵ����������ֵԼΪ��������
| ���� |
| A��52.6% | B��77.8% |
| C��80.7% | D��100% |
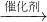 4NO+6H2O�� 2NO+O2=2NO2����3NO2+H2O=2HNO3+NO�����е�Ԫ��ȫ��ת��Ϊ������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O���ɴ˿��Ƴ�����ҵ����������������ԭ�ӵ����������ֵԼΪ
4NO+6H2O�� 2NO+O2=2NO2����3NO2+H2O=2HNO3+NO�����е�Ԫ��ȫ��ת��Ϊ������ܻ�ѧ����Ϊ��NH3+2O2�THNO3+H2O���ɴ˿��Ƴ�����ҵ����������������ԭ�ӵ����������ֵԼΪ