��Ŀ����
Ϊ�ⶨ��ʾ��������Ϊ32%�����ᣨͼ1����ʵ������������С����pH�ⶨ�����ʵ��װ�ã�ͼ2����ʵ��ʱ�����ձ��м���20g 40%������������Һ������μ�������ᣬ�ⶨ�Ǵ�ӡ������������������ձ�����ҺpH�Ĺ�ϵͼ��ͼ3����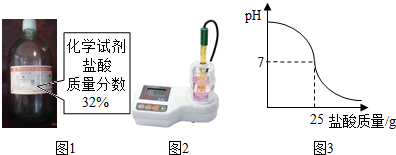
��1�����Դ˴βⶨ�Ľ��������������ʵ������������
��2���������ļ��������ǩ��ʾ������������һ�µĿ���ԭ��
��3����ȡ50g��������һ���ձ��У�����58.8gijŨ�ȵ�̼������Һǡ����ȫ��Ӧ�����ձ���������Һ����������������
��������1����������������ǡ�÷�Ӧʱ��PH=7��ͼ��֪����ʱ������Һ��������25g���������������Ƶ��������û�ѧ����ʽ�ļ����������������������
��2��Ũ�����ӷ�������ʱ�������ʹ������������������С��
��3���˷�Ӧ�����ɶ�����̼��ʹ��Һ������С�������÷���ʽ�ļ���������ʵ�����Ȼ��������
��2��Ũ�����ӷ�������ʱ�������ʹ������������������С��
��3���˷�Ӧ�����ɶ�����̼��ʹ��Һ������С�������÷���ʽ�ļ���������ʵ�����Ȼ��������
����⣺��1����������ʵ����������Ϊx��
HCl+NaOH=NaCl+H2O
36.5 40
25g x 20g��40%
=
x=29.2%
�𣺸������ʵ������������29.2%��
��2��Ũ�����ӷ�������ʱ�������ʹ��ᷢ���Ȼ��������ʹ������������������С��
�ʴ�Ϊ��Ũ������лӷ��ԣ����ڷ�������������С��
��3���裺�����Ȼ��Ƶ�������y��������̼��������z
2HCl+Na2CO3=2NaCl+H2O+CO2��
73 117 44
50g��29.2% y z
=
=
y=23.4g z=8.8 g ����NaCl��������
��100%=23.4%
���ձ���������Һ����������������23.4%
HCl+NaOH=NaCl+H2O
36.5 40
25g x 20g��40%
| 36.5 |
| 25gx |
| 40 |
| 20g��40% |
x=29.2%
�𣺸������ʵ������������29.2%��
��2��Ũ�����ӷ�������ʱ�������ʹ��ᷢ���Ȼ��������ʹ������������������С��
�ʴ�Ϊ��Ũ������лӷ��ԣ����ڷ�������������С��
��3���裺�����Ȼ��Ƶ�������y��������̼��������z
2HCl+Na2CO3=2NaCl+H2O+CO2��
73 117 44
50g��29.2% y z
| 73 |
| 50g��29.2% |
| 117 |
| y |
| 44 |
| z |
y=23.4g z=8.8 g ����NaCl��������
| 23.4g |
| 50g+58.8g-8.8g |
���ձ���������Һ����������������23.4%
�����������Ƕ���Һ������ʽ������Ŀ��飬�ҵ���֪�����û�ѧ����ʽ�������������������������ʵ����������ļ��������⣬��ɴ��������ѧ���������⼰����������һ��������

��ϰ��ϵ�д�
�����Ŀ


