��Ŀ����
13��Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ������������������ʲ��μӷ�Ӧ���Ҳ�����ˮ����ij��ȤС���������ʵ�鷽�����ٳ�ȡ10gʯ��ʯ��Ʒ
�ڸ����������������ٸı�
�۰�ʣ�������ڿ�������ȴ�����£��ܳƵ�ʣ����������Ϊ6.04g��
A�����в���������̼������Ϊ3.96gg��
B��������ʯ��ʯ��̼��Ƶ��������������ݻ�ѧ����ʽ��ʽ���㣩��
C����ʵ�鷽����һ�����ԵIJ��㲢˵��ԭ���ڿ�������ȴ�������ƻ�����ˮ�������������ƣ�
���� A�����������غ㶨�ɿ�֪���������ļ������������ɶ�����̼���������ö�����̼����������������Է��������������ʵ�����
B�����ݶ�����̼�����������̼��Ƶ��������ٳ���ʯ��ʯ���������ɣ�
C����Ϊ�����ƾ�����ˮ�����Բ����ڿ���������Ծݴ���ɽ��
��� �⣺A���ڷ����в���������̼������Ϊ��10g-6.04g=3.96g��
B����Ҫ����3.96g ������̼��Ҫ̼�������Ϊx��
CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2��
100 44
x 3.96g
$\frac{100}{x}$=$\frac{44}{3.96g}$
���x=9g
ʯ��ʯ��̼��Ƶ�����������$\frac{9g}{10g}$��100%=90%��
��ʯ��ʯ��̼��Ƶ���������90%��
����Ϊ�����ƾ�����ˮ�����Բ����ڿ�������ȴ��������������ˮ��Ӧ�����������ƣ������������ٵ����ˣ������
�ʴ�Ϊ����3.96g��
��90%��
���ڿ�������ȴ�������ƻ�����ˮ�������������ƣ�
���� �����Ĺؼ��ǹ��������ļ����������ɶ�����̼�����������ݶ�����̼�����̼��Ƶ�������Ҫ��������ƾ�����ˮ�ԣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �����ڲ����˶� | B�� | ����֮���м�� | C�� | ���Ӻ�С | D�� | ������ԭ�ӹ��� |
| A�� | Һ̬������ ������� | B�� | ����ˮ ����ʯ��ˮ | ||
| C�� | ��ˮ������ �ྻ�Ŀ��� | D�� | ˮ ��ˮ������ |
| A�� | CuSO4��Һ | B�� | H2O | C�� | ϡ���� | D�� | ϡ���� |
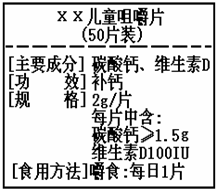 ��ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮
��ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮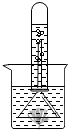 ��ͼ��֤ʵֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ���װ���뱭ˮ���ձ��ڷ���һЩ�����壬��ͨ��һ����������A������һ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ��������·���һ��ʱ�䣬�Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣮
��ͼ��֤ʵֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ���װ���뱭ˮ���ձ��ڷ���һЩ�����壬��ͨ��һ����������A������һ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ��������·���һ��ʱ�䣬�Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣮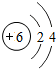 ��
��