��Ŀ����
��֪̼�����ȶ���̼�����ƣ�NaHC03�����ȶ��������ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3  Na2C03+C02��+H20����ȡ̼�����ƺ�̼���ƵĻ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ����
Na2C03+C02��+H20����ȡ̼�����ƺ�̼���ƵĻ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ����
- A.��Ӧ������C02������Ϊ3.1g
- B.ԭ�������NaHC03������Ϊ4.2g
- C.��Ӧ������C02��H20����������22��9
- D.ԭ�������Na2C03����������Ϊ16%
CD
����������NaHCO3����Na2CO3��H2O��CO2���ڼ���270��ʱ��H2O��CO2һ���������ɢʧ�ˣ������ٵ������������ɵ�CO2��H2O������֮�ͣ�
��𣺴˷�Ӧ����ˮ�Ͷ�����̼��������Ϊ10g-6.9g=3.1g
��ԭ�������NaHC03������Ϊm�����ɵ�H2O������Ϊx�����ɵ�CO2������Ϊ3.1g-x��
2NaHCO3 Na2CO3+H2O��+CO2����m
Na2CO3+H2O��+CO2����m
168 18 44 62
m x y 3.1g
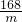 =
=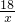 =
=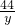 =
=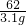
��֮Ϊ��x=0.9g y=2.2g��m=8.4g
�����ɵ�CO2������Ϊ��2.2g
��CO2��H2O����������9��22��
ԭ�������̼���Ƶ���������Ϊ��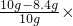 100%=16%��
100%=16%��
��ѡCD��
������Ҫ����ھ���Ŀ�е����������ǵ�Ӱ�����������仯�ĸ������أ���ų����塢���ɳ���������ˮ�ȣ�
����������NaHCO3����Na2CO3��H2O��CO2���ڼ���270��ʱ��H2O��CO2һ���������ɢʧ�ˣ������ٵ������������ɵ�CO2��H2O������֮�ͣ�
��𣺴˷�Ӧ����ˮ�Ͷ�����̼��������Ϊ10g-6.9g=3.1g
��ԭ�������NaHC03������Ϊm�����ɵ�H2O������Ϊx�����ɵ�CO2������Ϊ3.1g-x��
2NaHCO3
 Na2CO3+H2O��+CO2����m
Na2CO3+H2O��+CO2����m168 18 44 62
m x y 3.1g
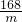 =
=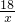 =
=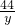 =
=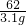
��֮Ϊ��x=0.9g y=2.2g��m=8.4g
�����ɵ�CO2������Ϊ��2.2g
��CO2��H2O����������9��22��
ԭ�������̼���Ƶ���������Ϊ��
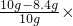 100%=16%��
100%=16%����ѡCD��
������Ҫ����ھ���Ŀ�е����������ǵ�Ӱ�����������仯�ĸ������أ���ų����塢���ɳ���������ˮ�ȣ�

��ϰ��ϵ�д�
�����Ŀ
��֪̼�����ȶ���̼�����ƣ�NaHC03�����ȶ��������ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3
Na2C03+C02��+H20����ȡ̼�����ƺ�̼���ƵĻ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ�������
| ||
| A����Ӧ������C02������Ϊ3.1g |
| B��ԭ�������NaHC03������Ϊ4.2g |
| C����Ӧ������C02��H20����������22��9 |
| D��ԭ�������Na2C03����������Ϊ16% |
��֪̼�����ȶ���̼�����ƣ�NaHCO3�����ȶ��������ֽ⣨2NaHCO3
Na2CO3+CO2��+H2O������ȡ̼�����ƺ�̼���ƵĻ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ�������
| ||
| A����Ӧ������CO2������Ϊ3.1g |
| B��ԭ�������Na2CO3����������Ϊ16% |
| C����Ӧ������CO2��H2O��������Ϊ22��9 |
| D��ԭ�������NaHCO3������Ϊ1.6g |
��֪̼�����ȶ���̼�����ƣ�NaHCO3�����ȶ��������ֽ⣨2NaHCO3
Na2CO3+CO2��+H2O������ȡ̼�����ƺ�̼���ƵĻ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ�������
| �� |
| A����Ӧ������CO2������Ϊ3.1g |
| B��ԭ�������NaHCO3������Ϊ1.6g |
| C����Ӧ������CO2��H2O��������Ϊ11��9 |
| D��ԭ�������Na2CO3����������Ϊ16% |