��Ŀ����
19��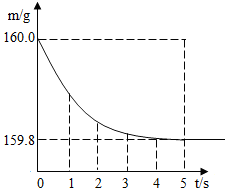 С��Ҫ�ⶨijCu-Zn�Ͻ���ͭ��������������������ʵ�飮
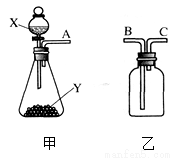
С��Ҫ�ⶨijCu-Zn�Ͻ���ͭ��������������������ʵ�飮��1������Ͳ��ȡ10.9mL 98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����100g 19.6%��ϡ���ᣮ
��2����������ɵ�ϡ����ȫ������ʢ��10g�Ͻ���Ʒ���ձ��У���Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ
�䣨t���Ĺ�ϵ��ͼ��ʾ��
�ٸúϽ���Ʒ��ͭ����������Ϊ35%��
�ڷ�Ӧ��������Һ������п������������ ��д�������̣���ȷ��0.1%��
���� ��1��������Һϡ���������ʵ��������ֲ�����м��㣬ע���������Ϊ�����
��2������ͼ���������ļ�����Ϊ���ɵ�������������������������������Ͷ�Ӧ�Ļ�ѧ����ʽ����п������������п��������Ȼ�������Ӧ������������
��� �⣺
��1������Ҫ��98%��Ũ���������Ϊx��
������Һϡ���������ʵ���������ɵ�
98%x=100g��19.6%
x=20g
�����Ϊ$\frac{20g}{1.84g/mL}$��10.9mL
��2�����������غ㶨�ɿɵã����ɵ�����������Ϊ160.0g-159.8g=0.2g
��μӷ�Ӧ��п������Ϊx�����ɵ�����п������Ϊy��
Zn+H2SO4=ZnSO4+H2��
65 161 2
x y 0.2g
$\frac{65}{x}$=$\frac{161}{y}$=$\frac{2}{0.2g}$
x=6.5g
y=16.1g
��Ͻ���ͭ����������Ϊ$\frac{10.0g-6.5g}{10.0g}$��100%=35%
������Һ������п����������Ϊ$\frac{16.1g}{6.5g+100g}$��100%��15.1%
�𣺣�1������Ͳ��ȡ 10.9mL 98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����100g 19.6%��ϡ���ᣮ
��2���ٸúϽ���Ʒ��ͭ����������Ϊ 35%��
�ڷ�Ӧ��������Һ������п����������Ϊ15.1%��
���� ���ݻ�ѧ����ʽ��������Ҫȷ������ֱ�����ڻ�ѧ����ʽ��������ݣ�����������������ֱ�����ڻ�ѧ����ʽ�ļ��㣮

| A�� | NO | B�� | N2 | C�� | NO2 | D�� | N2O |
| A�� | ˮϴ�Թ��ڵĸ������ | B�� | ϴ�ྫϴ�� | ||
| C�� | ϴ��ˮϴͷ | D�� | ����ϴ�·� |
| ѡ�� | ���� | ���� | ���ӷ��� |
| A | CO2 | CO | ͨ��������ȼ |
| B | CaO | CaCO3 | ��ˮ����� |
| C | Cu | Fe | ��������ϡ�������� |
| D | KCl | KClO3 | �Ӷ������̼��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���������ױ��� | B�� | Ũ��ˮ�ӷ� | ||
| C�� | Ũ���������ˮ�� | D�� | ������������� |
| A�� | ��ҵ��ˮֱ��������� | |
| B�� | �ᳫʹ��ú̿��ʯ�͵Ȼ�ʯȼ�� | |
| C�� | �ƹ�ʹ�÷��ܣ�̫���ܵ�����Դ | |
| D�� | ����ʹ��ũҩ���������ũ������� |
 ��ͼժ��Ԫ�����ڱ����ݴ��ж���������������ǣ�������
��ͼժ��Ԫ�����ڱ����ݴ��ж���������������ǣ�������| A�� | ����Ԫ�ض����ڷǽ���Ԫ�� | |
| B�� | �������ԭ������Ϊ16.00 | |
| C�� | C��N��O��ԭ���������ε��� | |
| D�� | ̼����������Ԫ�����ڱ�������ͬһ�� |