��Ŀ����
Ϊ�˲ⶨij��ͭ��ͭп�Ͻ���Ʒ����ɣ�ij��ѧ��ȤС���ͬѧ����������ʵ�飺ȡ�ķ���ͬ��������Ʒ�ֱ������������ձ��У�Ȼ��ֱ����ϡ���ᣬ��ַ�ӳ������ƽ���������������������£�
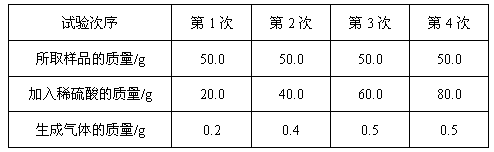
��ش��������Ⲣ���㣺
��1�����������ڵ�1��ʵ�������Ӧ��_________��ȫ��Ӧ���ˡ�
��2��50.0g��Ʒ������ϡ���ᷴӦ�������������_________g��
��3��������Ʒ��п������������д�����������̣���
��4������ͼ�л�������ϡ�������������������������ı仯��ϵ��
��1�����������ڵ�1��ʵ�������Ӧ��_________��ȫ��Ӧ���ˡ�
��2��50.0g��Ʒ������ϡ���ᷴӦ�������������_________g��
��3��������Ʒ��п������������д�����������̣���
��4������ͼ�л�������ϡ�������������������������ı仯��ϵ��
��1�����
��2��0.5��
��3���⣺��50g��Ʒ��п������Ϊx��
Zn��H2SO4��ZnSO4��H2��
65 2
x 0.5g
65��x��2��0.5g
x��16.25g
��Ʒ��п����������Ϊ��16.25g/50g ��100%��32.5%��
����Ʒ��п����������Ϊ32.5%��
��4������ϡ�������������������������ı仯��ϵͼΪ��
��2��0.5��
��3���⣺��50g��Ʒ��п������Ϊx��
Zn��H2SO4��ZnSO4��H2��
65 2
x 0.5g
65��x��2��0.5g
x��16.25g
��Ʒ��п����������Ϊ��16.25g/50g ��100%��32.5%��
����Ʒ��п����������Ϊ32.5%��
��4������ϡ�������������������������ı仯��ϵͼΪ��
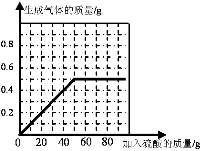

��ϰ��ϵ�д�
�����Ŀ
Ϊ�˲ⶨij��ͭ��ͭп�Ͻ���п������������ij������ȤС�����øúϽ���ʵ�����е�һƿϡ���ᷴӦ�����������ɴ�ʵ�飮�ֽ����е�����ʵ������ժ¼���£�
��ش��������⣺
��1���� ��ʵ�飬��ͭ�е�п��ϡ����ǡ����ȫ��Ӧ��
��2�������ͭ��п������������д��������̣���
| ʵ����� | 1 | 2 | 3 |
| ϡ�����������g�� | 100 | 100 | 100 |
| �����ͭ��ͭп�Ͻ𣩵�������g�� | 6.5 | 13 | 19.5 |
| ��������������g�� | 0.1 | 0.2 | 0.2 |
��1����
��2�������ͭ��п������������д��������̣���
 ��2013?����ģ�⣩Ϊ�˲ⶨij��ͭ��ͭп�Ͻ���Ʒ����ɣ�ij��ѧ��ȤС���ͬѧ����������ʵ�飺ȡ�ķ���ͬ��������Ʒ�ֱ������������ձ��У�Ȼ��ֱ����ϡ���ᣬ��ַ�ӳ������ƽ���������������������£�
��2013?����ģ�⣩Ϊ�˲ⶨij��ͭ��ͭп�Ͻ���Ʒ����ɣ�ij��ѧ��ȤС���ͬѧ����������ʵ�飺ȡ�ķ���ͬ��������Ʒ�ֱ������������ձ��У�Ȼ��ֱ����ϡ���ᣬ��ַ�ӳ������ƽ���������������������£�