��Ŀ����
ѧУ������ȤС���ͬѧ��Ϊ�ⶨij������Ʒ���ٶ�����ֻ���������͵���̼���������������������Ǹ���ȡ10g������Ʒ�ֱ�ŵ���ͬŨ�ȡ���ͬ������ϡ�����У���ַ�Ӧ�ֱ�õ��������ݣ�
| ʵ����� | 1 | 2 | 3 | 4 |
| ϡ��������/g | 25 | 50 | 75 | 100 |
| ����H2������/g | 0.1 | 0.2 | 0.3 | 0.3 |
��2����Ҫ������������100t����Ҫ��Fe2O380%�ij�������ٶ֣�
�⣺��1�����ݱ��е����ݼ���75gϡ������100gϡ�������ɵ�������������0.3g�����Ը���Ʒ���������������������0.3g��
����������������Ϊx����
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3g

x=8.4g
���������Ʒ��������������Ϊ�� ��100%=84%
��100%=84%
��2������Ҫ��Fe2O380%�ij�����������y
100t��84%=y��80%��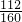
y=150t
�𣺣�1����������Ʒ��������������Ϊ84%��
��2����Ҫ������������100t����Ҫ��Fe2O380%�ij�����150�֣�
��������1�����ݱ��е����ݼ���75gϡ������100gϡ�������ɵ�������������0.3g�����Ը���Ʒ���������������������0.3g�������йصĻ�ѧ����ʽ���ɼ���������������Ӷ��ɵ���������������
��2������ұ����������Ԫ�ص���������ı��ԭ�������û�ѧʽ�ļ����ɣ�
�����������Ĺؼ��Ǹ���ͼ���е������ж�������������������ٽ��н�һ�����㣮
����������������Ϊx����
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3g

x=8.4g
���������Ʒ��������������Ϊ��
 ��100%=84%
��100%=84%��2������Ҫ��Fe2O380%�ij�����������y
100t��84%=y��80%��
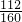
y=150t
�𣺣�1����������Ʒ��������������Ϊ84%��
��2����Ҫ������������100t����Ҫ��Fe2O380%�ij�����150�֣�
��������1�����ݱ��е����ݼ���75gϡ������100gϡ�������ɵ�������������0.3g�����Ը���Ʒ���������������������0.3g�������йصĻ�ѧ����ʽ���ɼ���������������Ӷ��ɵ���������������
��2������ұ����������Ԫ�ص���������ı��ԭ�������û�ѧʽ�ļ����ɣ�
�����������Ĺؼ��Ǹ���ͼ���е������ж�������������������ٽ��н�һ�����㣮

��ϰ��ϵ�д�
�����Ŀ
ѧУ������ȤС���ͬѧ��Ϊ�ⶨij������Ʒ���ٶ�����ֻ���������͵���̼���������������������Ǹ���ȡ10g������Ʒ�ֱ�ŵ���ͬŨ�ȡ���ͬ������ϡ�����У���ַ�Ӧ�ֱ�õ��������ݣ�
��1���Լ����������Ʒ����������������
��2����Ҫ������������100t����Ҫ��Fe2O380%�ij�������ٶ֣�
| ʵ����� | 1 | 2 | 3 | 4 |
| ϡ��������/g | 25 | 50 | 75 | 100 |
| ����H2������/g | 0.1 | 0.2 | 0.3 | 0.3 |
��2����Ҫ������������100t����Ҫ��Fe2O380%�ij�������ٶ֣�
