��Ŀ����
ͬѧ�Ǵ�ɽ�ϲɼ���һ��ʯ��ʯ(��Ҫ�ɷ�Ϊ̼���),����ȡ80 g����Ʒ��������ʵ��(�������������չ����в������仯),��÷�Ӧ�����������뷴Ӧʱ��Ĺ�ϵ���±�:
| ��Ӧʱ��/s | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
| ��Ӧ������ ����/g | 80 | 75 | 70 | 66 | 62 | 58 | 58 |
��ش���������:
(1)��ʯ��ʯ��ȫ��Ӧ��,����CO2������Ϊ ��__________��
��__________��
(2)���ʯ��ʯ�к�CaCO3����������(д���������)��
������������ͼ�б����֪��t5ʱ�����������ټ���,��֪̼�����ȫ�ֽ�,���������غ㶨�����ɶ�����̼������Ϊ80 g-58 g=22 g,����̼��Ʒֽ�Ļ�ѧ����ʽ,��̼����������̼��������ϵ,���ݶ�����̼���������Լ���̼��Ƶ�����,�Ӷ����Լ���̼��Ƶ�����������
��:(1)22 g
(2)����Ʒ�к�CaCO3������Ϊx
CaCO3 CaO+CO2��
CaO+CO2��
100 44
x 2 2 g
2 g
 =
=
x= =50 g
=50 g
��Ʒ��CaCO3����������= ��100%=62.5%
��100%=62.5%
��:��Ʒ��CaCO3����������Ϊ62.5%��

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ

 2R+O2���ṩ����,����R�Ļ�ѧʽ�ǡ�(����)
2R+O2���ṩ����,����R�Ļ�ѧʽ�ǡ�(����)
 ___________________��
___________________��
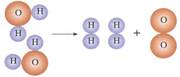 A���ڻ�ѧ�仯��ԭ������С������
A���ڻ�ѧ�仯��ԭ������С������