��Ŀ����
9�� ��ͼ��ʾ��A��F�dz��л�ѧ���������ʣ���֪E������������Է���������С�����ʣ�C��D��Ӧ�ɲ������壬�Իش��������⣺
��ͼ��ʾ��A��F�dz��л�ѧ���������ʣ���֪E������������Է���������С�����ʣ�C��D��Ӧ�ɲ������壬�Իش��������⣺��1��E����;�����
��2����C��D��Ӧ��ʵ������ȡCO2�ķ�Ӧԭ������A��B��Ӧ�����������Dz�����ɫ������B��C��Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+2HCl=CaCl2+2H2O��
��3����C��D��Ӧ������Ϊ��ɫ��Һ���dz��ɫ����B��������A��
A������������ B���ǽ��������� C���� D���� E���Σ�
���� ����A��F�dz��л�ѧ���������ʣ�E������������Է���������С�����ʣ�����E��ˮ��C��D��Ӧ�ɲ������壬����B�������������ƣ�A�Ƕ�����̼��D��̼���ƣ�E��ˮ��C���������ᣬ����F���Ȼ��ƣ�Ȼ���Ƴ������ʽ�����֤���ɣ�
��� �⣺��1��A��F�dz��л�ѧ���������ʣ�E������������Է���������С�����ʣ�����E��ˮ��C��D��Ӧ�ɲ������壬����B�������������ƣ�A�Ƕ�����̼��D��̼���ƣ�E��ˮ��C���������ᣬ����F���Ȼ��ƣ�������֤���Ƶ���ȷ������E��ˮ�������������
��2����C��D��Ӧ��ʵ������ȡCO2�ķ�Ӧԭ��������C�����ᣬD��̼��ƣ���A��B��Ӧ�����������Dz�����ɫ������B��C�ķ�Ӧ���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ����ѧ����ʽΪ��Ca��OH��2+2HCl=CaCl2+2H2O��
��3����C��D��Ӧ������Ϊ��ɫ��Һ���dz��ɫ������C������D���Ȼ�ͭ��A��B�ķ�Ӧ����������ͭ�����ᷴӦ�����Ȼ�ͭ��ˮ������B�������ڽ����������ѡ��A��
�ʴ�Ϊ����1�����
��2��������ɫ������Ca��OH��2+2HCl=CaCl2+2H2O��
��3��A��
���� �ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ������ʺ����е�ת����ϵ�Ƶ�ʣ������ʣ�����Ƴ��ĸ������ʴ���ת����ϵ�н�����֤���ɣ�

 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�| A�� | ���ʱ������������� | B�� | �ᳫ��ɫ���У���չ������ͨ | ||
| C�� | ֲ���̻����������� | D�� | ����ú̿���磬����̫���ܷ��� |
| A�� | ��ͷ | B�� | ��ţ�� | C�� | ���� | D�� | ���ܲ� |
| A�� | �ǻ���ʯ������ | |
| B�� | �����ͻ�ֿ���Ϊ��ȼ���Ϻ����²��ϵ� | |
| C�� | �ǻ���ʯ���⡢��Ԫ��������Ϊ1��208 | |
| D�� | �ǻ���ʯ�к���10���ԭ�ӣ�2����ԭ�ӣ�26����ԭ�ӣ�6����ԭ�ӣ� |
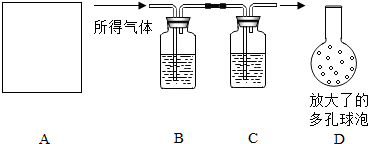 Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ��
Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ��


 ˮ������֮Դ�������о���ˮ�ķ����࣮ܶ
ˮ������֮Դ�������о���ˮ�ķ����࣮ܶ