��Ŀ����
8��ʯ�ҳ�Ϊ�ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ4gʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ�У���Ʒ�е������ɷּȲ������ᷴӦ��Ҳ������ˮ������ַ�Ӧ���ˡ���������Ȳ�����ʵ�����������| ʵ�� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ϡ��������� | 5g | 5g | 5g | 5g |
| ʣ���������� | X | 2g | 1g | 1g |
��2����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
��3������ʵ������в���������̼�������������
���� ��1�����ݵ����μ������������������1g�����Ե�һ�μ��������������Ҳ����1g������m��ֵ��4g-1g=3g���з�����
��2��������Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦ��Ҳ������ˮ����˵����Ρ����Ĵ�ʵ����ʣ��Ĺ��弴��������ӦҲ������ˮ�����ʹ�����������з�����
��3�����ݻ�ѧ����ʽ�Ͳμӷ�Ӧ��̼��Ƶ��������м��㣮
��� �⣺��1�������μ������������������1g�����Ե�һ�μ��������������Ҳ����1g������m��ֵ��4g-1g=3g��
��2����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��$\frac{4g-1g}{4g}$��100%=75%��
��3�������������̼���������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
3g x
$\frac{100}{3g}$=$\frac{44}{x}$
x=1.32g
��ʵ������в���������̼�����������Ϊ1.32g��
�ʴ�Ϊ����1��3��
��2��75%��
��3��ʵ������в���������̼�����������Ϊ1.32g��
���� ������Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�

| A�� | �����ۻ� | B�� | ���ͻӷ� | C�� | ֽ��ȼ�� | D�� | ��ˮɹ�� |
| A�� | -1 | B�� | +1 | C�� | +5 | D�� | +7 |
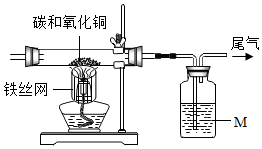 ѧϰ̼���ʵ�����ʱ��ijУʵ��С���ͬѧΪ̽��̼�Ļ�ѧ���ʣ��������ʵ��װ�ö�ľ̿������ͭ�ķ�Ӧ������ʵ��̽������������������⣮
ѧϰ̼���ʵ�����ʱ��ijУʵ��С���ͬѧΪ̽��̼�Ļ�ѧ���ʣ��������ʵ��װ�ö�ľ̿������ͭ�ķ�Ӧ������ʵ��̽������������������⣮��1��Һ��M�dz����ʯ��ˮ��
��2���ƾ��Ƽ�����˿���ֵ�����������¶ȣ�
��3�����������
| ʵ������ | �û�ѧ����ʽ���ʹ����� |
| �������ں�ɫ��ĩ��� | C+2CuO$\frac{\underline{\;����\;}}{\;}$2Cu+CO2�� |
| �ڳ����ʯ��ˮ����� | Ca��OH��2+CO2�TCaCO3��+H2O |
��5�������Һ��M������ɫʯ����Һ��ʵ��ʱ�ῴ����ɫʯ����Һ��죬��صĻ�ѧ����ʽCO2+H2O�TH2CO3��
| A�� | NaOH��HCl | B�� | Fe2O3��HCl | C�� | BaCl2��H2SO4 | D�� | NaOH��CO2 |
| A�� | ���ʯ����������ͭ�����ڽ������� | |
| B�� | �ϳɲ��ϰ����ϳ���ά���ϳ����Ͻ� | |
| C�� | ���ó�˿�����ա�����ζ�ķ���������ë��ά������ά | |
| D�� | ʯī��������Ϊ���� |
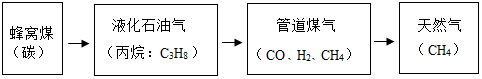
 ��ͼ��ʾ���dz������ճ�������Ʒ���������л��߷��Ӳ��ϵ��У��ۢޣ�����ţ���������Ҫ������ë���ͺϳ���ά����ͨ��ȼ�շ��ķ�����
��ͼ��ʾ���dz������ճ�������Ʒ���������л��߷��Ӳ��ϵ��У��ۢޣ�����ţ���������Ҫ������ë���ͺϳ���ά����ͨ��ȼ�շ��ķ�����