��Ŀ����
��������ƣ�ijͬѧ������һ���Ȼ��Ʒ�ĩ��һ��̼���Ʒ�ĩ������һ��ij�о�С���ͬѧ��̽����ݷ�ĩ�и���ֵĺ�����������������ʵ�飺
�ٽ���ĩ��Ͼ��ȣ�ȡ10g��ĩ���������45.7gˮ���Ƴɻ����Һ��
��������Һ����εμ�������������Ϊ10%��ϡ���ᣬ����������Ϊ
�۽�����������ȫ����ͨ��ʢ��Ũ�����ϴ��ƿ��Ŀ����Ϊ��
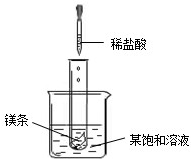
�ܻ�������������Һ������������������ϡ���������Ĺ�ϵ���ߣ���ͼ2��
�ش��������⣺
��1�����ʵ�鲽��ڡ��۵������հף�
��2����Ӧ�����У����ɶ�����̼������Ϊ
��3������ ��ԭ��Ϸ�ĩ���Ȼ��Ƶ������� �ڵ���Ӧǡ�ý���ʱ��������Һ��������������Ϊ���٣�������һλС����
�ٽ���ĩ��Ͼ��ȣ�ȡ10g��ĩ���������45.7gˮ���Ƴɻ����Һ��
��������Һ����εμ�������������Ϊ10%��ϡ���ᣬ����������Ϊ
������������
������������
�۽�����������ȫ����ͨ��ʢ��Ũ�����ϴ��ƿ��Ŀ����Ϊ��
��ȥ������̼�����е�ˮ����
��ȥ������̼�����е�ˮ����
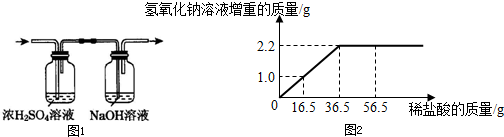
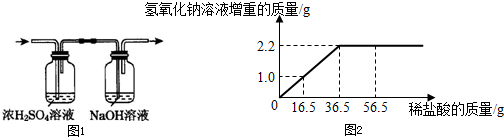
�����ս�����ͨ����������������Һ�У���ͼ1������������������Һ�����أ����������Բ��ƣ����ܻ�������������Һ������������������ϡ���������Ĺ�ϵ���ߣ���ͼ2��

�ش��������⣺
��1�����ʵ�鲽��ڡ��۵������հף�
��2����Ӧ�����У����ɶ�����̼������Ϊ
2.2
2.2
g����3������ ��ԭ��Ϸ�ĩ���Ȼ��Ƶ������� �ڵ���Ӧǡ�ý���ʱ��������Һ��������������Ϊ���٣�������һλС����
��������1������̼�����������ᷴӦ���ɶ�����̼�Լ�Ũ���᳤��������������
��2������ͼ�������ص�����������
��3�����ݶ�����̼���������û�ѧ����ʽ���̼���Ƶ��������ɽ���⣻
��2������ͼ�������ص�����������
��3�����ݶ�����̼���������û�ѧ����ʽ���̼���Ƶ��������ɽ���⣻
����⣺��1������̼�����������ᷴӦ���ɶ�����̼�����Ըù��̵������Dz����������ݣ�
Ũ�����dz����ĸ�������˴����ǶԶ�����̼���и���Ĺ��̣��������dz�ȥ������̼�����е�ˮ������
��2����ͼ���֪��Һ������2.2g�������ӣ��������ɶ�����̼��������2.2g��
��3����ԭ��Ϸ�ĩ��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117 44
x y 2.2g
=
=
x=5.3g y=5.85g
ԭ��Ϸ�ĩ���Ȼ��Ƶ�����Ϊ 10g-5.3g=4.7g
ǡ����ȫ��Ӧʱ������Һ��������������Ϊ
��100%=11.7%
�ʴ�Ϊ����1�������������ݣ���ȥ������̼�����е�ˮ��������2��2.2��
��3������ ��ԭ��Ϸ�ĩ���Ȼ��Ƶ�����Ϊ4.7g�� �ڵ���Ӧǡ�ý���ʱ��������Һ��������������Ϊ11.7%��
Ũ�����dz����ĸ�������˴����ǶԶ�����̼���и���Ĺ��̣��������dz�ȥ������̼�����е�ˮ������
��2����ͼ���֪��Һ������2.2g�������ӣ��������ɶ�����̼��������2.2g��
��3����ԭ��Ϸ�ĩ��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117 44
x y 2.2g
| 106 |
| x |
| 117 |
| y |
| 44 |
| 2.2g |
x=5.3g y=5.85g
ԭ��Ϸ�ĩ���Ȼ��Ƶ�����Ϊ 10g-5.3g=4.7g
ǡ����ȫ��Ӧʱ������Һ��������������Ϊ
| 4.7g+5.85g |
| 10g+45.7g+36.5g-2.2g |
�ʴ�Ϊ����1�������������ݣ���ȥ������̼�����е�ˮ��������2��2.2��
��3������ ��ԭ��Ϸ�ĩ���Ȼ��Ƶ�����Ϊ4.7g�� �ڵ���Ӧǡ�ý���ʱ��������Һ��������������Ϊ11.7%��
�����������㿼����ʵ����ƺ��йصļ����⣬�����й�ʵ������ԭ���ͷ�Ӧ�Ĺ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
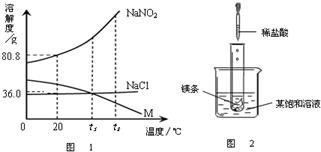
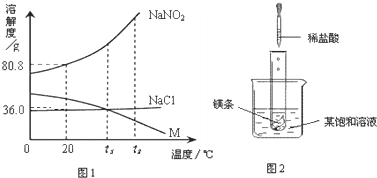
 ��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺
��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺