��Ŀ����
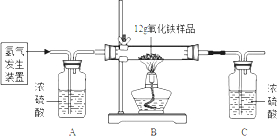 ijͬѧΪ�ⶨ12g�����ʵ���������Ʒ������������������������ϡ�����п����ȡ�������������ͼ��ʾ��װ�ã������йص�ʵ��̽������ʾ��3H2+Fe2O3
ijͬѧΪ�ⶨ12g�����ʵ���������Ʒ������������������������ϡ�����п����ȡ�������������ͼ��ʾ��װ�ã������йص�ʵ��̽������ʾ��3H2+Fe2O3
| ||
���ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ���
�õ��������ݣ�
| ������Ŀ | ��Ӧǰ���� | ��Ӧ������ |
| Aϴ��ƿ����ʢŨ��������� | 290��3g | 291��5 g |
| B�����ܼ���ʢ��������� | 86��3g | 83��9g |
| Cϴ��ƿ����ʢŨ��������� | 284��2g | 286.9g |
��2����ʵ�黹�ɲⶨ���ˮ�ĸ�Ԫ��֮���������ϵ�����ñ���ʵ��������ʽ��ʾ��ˮ���⡢��Ԫ�ص������ȣ���ʽΪ
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1��Cװ��ΪŨ���ᣬ���շ�Ӧ���ɵ�ˮ����Cװ�����ӵ������������ɵ�ˮ��������Ȼ����ݻ�ѧ����ʽ������ˮ�����������������������Ȼ������������������������
��2������Bװ���м��ٵ�����Ϊ��Ԫ�ص��������ָ���Cװ�������ӵ�����Ϊˮ��������ˮ����Ԫ�غ���Ԫ����ɣ���ˮ��������ȥ��Ԫ�ص�����������Ԫ�ص��������ʶ����������Ԫ�ص������ȣ����ʵ������һ���ģ�ˮ������Ԫ�ص���������һ���ģ��ʼ�ʹ�������е�������û����ȫ��Ӧ��ˮ������Ԫ�ص��������Dz���ģ�
��2������Bװ���м��ٵ�����Ϊ��Ԫ�ص��������ָ���Cװ�������ӵ�����Ϊˮ��������ˮ����Ԫ�غ���Ԫ����ɣ���ˮ��������ȥ��Ԫ�ص�����������Ԫ�ص��������ʶ����������Ԫ�ص������ȣ����ʵ������һ���ģ�ˮ������Ԫ�ص���������һ���ģ��ʼ�ʹ�������е�������û����ȫ��Ӧ��ˮ������Ԫ�ص��������Dz���ģ�
����⣺��1������ˮ������Ϊ286.9g-284.2g=2.7g
����Ʒ��������������ΪX
3H2+Fe2O3
2Fe+3H2O
160 3��18
x 2.7g
=
x=8g
��Ʒ������������������Ϊ��
��100%��66.7%
����Ʒ������������������Ϊ66.7%
��2������Bװ���м��ٵ�����Ϊ��Ԫ�ص���������86.3g-83.9g���ָ���Cװ�������ӵ�����Ϊˮ��������ˮ����Ԫ�غ���Ԫ����ɣ���ˮ��������ȥ��Ԫ�ص�����������Ԫ�ص�����������286.9g-284.2g��-��86.3g-83.9g��������Ԫ�غ���Ԫ�ص�������Ϊ������286.9g-284.2g��-��86.3g-83.9g��������86.3g-83.9g����
��Ϊ���ʵ������һ���ģ�ˮ������Ԫ�ص���������һ���ģ��ʼ�ʹ�������е�������û����ȫ��Ӧ��ˮ������Ԫ�ص��������Dz���ģ�
�ʴ�Ϊ����1����Ʒ������������������Ϊ66.7%��
��2������286.9g-284.2g��-��86.3g-83.9g��������86.3g-83.9g�������䣻
����Ʒ��������������ΪX
3H2+Fe2O3
| ||
160 3��18
x 2.7g
| 160 |
| x |
| 3��18 |
| 2.7g |
x=8g
��Ʒ������������������Ϊ��
| 8g |
| 12g |
����Ʒ������������������Ϊ66.7%
��2������Bװ���м��ٵ�����Ϊ��Ԫ�ص���������86.3g-83.9g���ָ���Cװ�������ӵ�����Ϊˮ��������ˮ����Ԫ�غ���Ԫ����ɣ���ˮ��������ȥ��Ԫ�ص�����������Ԫ�ص�����������286.9g-284.2g��-��86.3g-83.9g��������Ԫ�غ���Ԫ�ص�������Ϊ������286.9g-284.2g��-��86.3g-83.9g��������86.3g-83.9g����
��Ϊ���ʵ������һ���ģ�ˮ������Ԫ�ص���������һ���ģ��ʼ�ʹ�������е�������û����ȫ��Ӧ��ˮ������Ԫ�ص��������Dz���ģ�
�ʴ�Ϊ����1����Ʒ������������������Ϊ66.7%��
��2������286.9g-284.2g��-��86.3g-83.9g��������86.3g-83.9g�������䣻
����������������ʵ�����ݷ�����Bװ�ü��ٵ���������Ԫ�ص�������Cװ�����ӵ������Ƿ�Ӧ���ɵ�ˮ�����������ǽ������Ĺؼ���

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ
���г�������ı仯�У����ڻ�ѧ�仯���ǣ�������
| A��ˮ�ķ��� |
| B���ƹ��гɱ�Ƭ |
| C����Ȼ����ȼ�� |
| D�����ͻӷ� |
�����йز��ϡ���Դ��������ʳƷ��ȫ������ȷ���ǣ�������
| A�����úϳɲ���Ϊ���ϡ��ϳ����ϳ���ά |
| B����ʯȼ����ָľ̿�����͡����� |
| C��������ֻ����������β���ŷ���ɵ� |
| D����ʳƷ�м�������������ӳ������� |
��ȼ�������������ȷֽ�ʱ����������ͬʱ�������µ���������ˮ�������������ã����й��ڸ���ȼ������ԭ��������д�����ǣ�������
| A����Ӧ���ȣ������˿�ȼ����Ż�� |
| B����Ӧ�ܹ������¶ȣ���ȼ�ﲻ�״ﵽ�Ż�� |
| C�����ɴ���ˮ���������Ϳ�ȼ����Χ����Ũ�� |
| D�����������������ڿ�ȼ����棬�������� |
���й��ڷ��ӵ�˵������ȷ���ǣ�������
| A�����ʶ����ɷ��ӹ��ɵ� |
| B�������DZ���ijЩ���ʻ�ѧ���ʵ�һ������ |
| C�������DZ��������������ʵ���С���� |
| D�������ǹ������ʵ�һ������ |
 ĩ�˾ƾ��Ƶ�������
ĩ�˾ƾ��Ƶ�������