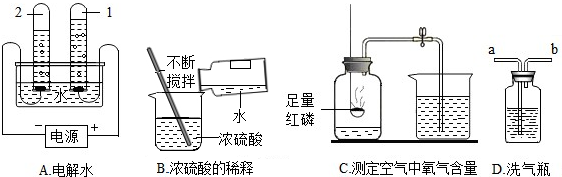
��Ŀ����
�밴Ҫ����գ�
��1����д����Ӧ�Ļ�ѧ���Ż����ƣ�
��2����ԭ��______����+2�۵�þԪ��______����2C1-______����H2O2��______��
��2�����H��C��O��Na��4��Ԫ����ѡ��ǡ����Ԫ�أ���ɷ�������Ҫ������ʣ������仯ѧʽ��д����Ӧ�Ŀո��ϣ�
����ȼ�յĹ�̬�ǽ���������______��
��һ���ж�����̬�������������������______��
������Ԫ����ɵ��Σ�����Һ�ʼ��ԣ���������ϴ�Ӽ�����______��
�ܲ���ֲ��Ĺ�����ã�������ũ����������ϵ���______��
������л������Ȼ������Ҫ�ɷ�______��
��1����д����Ӧ�Ļ�ѧ���Ż����ƣ�
��2����ԭ��______����+2�۵�þԪ��______����2C1-______����H2O2��______��
��2�����H��C��O��Na��4��Ԫ����ѡ��ǡ����Ԫ�أ���ɷ�������Ҫ������ʣ������仯ѧʽ��д����Ӧ�Ŀո��ϣ�
����ȼ�յĹ�̬�ǽ���������______��
��һ���ж�����̬�������������������______��
������Ԫ����ɵ��Σ�����Һ�ʼ��ԣ���������ϴ�Ӽ�����______��
�ܲ���ֲ��Ĺ�����ã�������ũ����������ϵ���______��
������л������Ȼ������Ҫ�ɷ�______��
��1���ٱ�ʾ���ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����ԭ�ӣ��Ϳɱ�ʾΪ2S��
��Ԫ�ػ��ϼ۵ı�ʾ�������ڸ�Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
��
������ʾ������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣���2C1-��ʾ��2�������ӣ�
�ܷ���H2O2��ʾ�����������˫��ˮ��
��2���������⣬��֪��ȼ�յĹ�̬�ǽ���������̼������ԭ��ֱ�ӹ��ɣ��ʻ�ѧʽΪC��
��һ����̼�Ļ�ѧʽ����ȶ�����̼�Ļ�ѧʽ����д�����ö���������������д�����仯ѧʽΪ��CO��
�۴���Ϊ̼���ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ�̼����Ļ��ϼ�Ϊ-2�ۣ���ע���ϼ�
�����û��ϼ���ֵ���淨��д��ѧʽNa2CO3��
�ܶ�����̼�Ļ�ѧʽ����д�����ö���������������д�����仯ѧʽΪ��CO2��
�ݼ���Ļ�ѧʽ�����л������д���ɣ����仯ѧʽΪ��CH4
�ʴ�Ϊ����1����2S����
����2�������ӣ��ܹ��������˫��ˮ����2����C����CO����Na2CO3����CO2����CH4
��Ԫ�ػ��ϼ۵ı�ʾ�������ڸ�Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
| +2 |
| Mg |
������ʾ������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣���2C1-��ʾ��2�������ӣ�
�ܷ���H2O2��ʾ�����������˫��ˮ��
��2���������⣬��֪��ȼ�յĹ�̬�ǽ���������̼������ԭ��ֱ�ӹ��ɣ��ʻ�ѧʽΪC��
��һ����̼�Ļ�ѧʽ����ȶ�����̼�Ļ�ѧʽ����д�����ö���������������д�����仯ѧʽΪ��CO��
�۴���Ϊ̼���ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ�̼����Ļ��ϼ�Ϊ-2�ۣ���ע���ϼ�
| +1 |
| Na |
| -2 |
| CO 3 |
�ܶ�����̼�Ļ�ѧʽ����д�����ö���������������д�����仯ѧʽΪ��CO2��
�ݼ���Ļ�ѧʽ�����л������д���ɣ����仯ѧʽΪ��CH4
�ʴ�Ϊ����1����2S����
| +2 |
| Mg |

��ϰ��ϵ�д�
�����Ŀ
�밴Ҫ����գ�
��1��ѡ������Ϥ�����ʣ��û�ѧʽ��д�ڶ�Ӧ�Ŀո���
��2�����š�NH4+������ ��
��3��д���ù���������������̻�������Ļ�ѧ��Ӧ����ʽ�� ��
��1��ѡ������Ϥ�����ʣ��û�ѧʽ��д�ڶ�Ӧ�Ŀո���
| ��� | ���� | ������ | �� | �� | �� | ��� |
| ��ѧʽ |
��3��д���ù���������������̻�������Ļ�ѧ��Ӧ����ʽ��
 �밴Ҫ����գ�
�밴Ҫ����գ�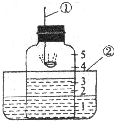 ��ͼ��ʾ���ô�װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��ͼ��ʾ���ô�װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�