��Ŀ����
���ݳ��л�ѧ��ѧ֪ʶ�ش��������⣺
��1��ʵ��������һ��������Ϊ98%����Ϊ1.84g/mL��Ũ��������46g��������Ϊ19.6%��ϡ���ᣬ�밴Ҫ����д�ո�
ij�������������������Ƶ�ϡ������ϴ��һ������Ʒ�в�����������ƣ�������ϡ���Ṳ30g��ϴ�Ӻ����Һ�����ԣ���һ������Ʒ�к��������Ƶ������Ƕ��٣������ԭ������---16 H---1 Na---23 S---32��
��1��ʵ��������һ��������Ϊ98%����Ϊ1.84g/mL��Ũ��������46g��������Ϊ19.6%��ϡ���ᣬ�밴Ҫ����д�ո�
| ʵ�鲽�� | ʵ������ |
| �ټ��� | Ũ��������Ϊ ˮ�����Ϊ ��д��������̣� |
| ����ȡ | ��Ͳ���� |
| ���ܽ� | һ��Ҫ�Ȱ� |
���㣺һ������������������Һ������,Ũ��������ʼ�Ũ�����ϡ��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��Һ����Һ���ܽ��
��������1������ϡ�͵���Һ�����ʵ�������ϡ��ǰ���ϡ��Ũ����Ļ����������н��
��2�������������������ᷴӦ�Ļ�ѧ����ʽ�������������Ƶ�������������������
��2�������������������ᷴӦ�Ļ�ѧ����ʽ�������������Ƶ�������������������
����⣺��1����Ũ��������Ϊx����
x?1.84g?mL-1��98%=46g��19.6%
���x=5mL��
��ҪŨ���������Ϊ1.84g?mL-1��5mL=9.2g
��Ҫˮ������Ϊ46g-9.2g=36.8gԼ37mL��
��ȡʱ��ͲҪ��ƽ������Ҫƽ�Ӱ�Һ�����ײ���ϡ��ʱҪ��Ũ�������ˮ�У����ܽ�ˮ����Ũ�����У����ò��������Ͻ��裻
�ʴ𰸣���5mL��37mL����ƽ�ţ���Һ�棻��ײ�����ˮ��Ũ�����������
��2�����ĵ�������Һ�����ʵ�����Ϊ��30g��20%=6g
��һ������Ʒ�к��������Ƶ�������x
2NaOH+H2SO4=Na2SO4+2H2O
80 98
x 6g
=
x=4.9g
��һ������Ʒ�к��������Ƶ�������4.9g��
x?1.84g?mL-1��98%=46g��19.6%
���x=5mL��
��ҪŨ���������Ϊ1.84g?mL-1��5mL=9.2g
��Ҫˮ������Ϊ46g-9.2g=36.8gԼ37mL��
��ȡʱ��ͲҪ��ƽ������Ҫƽ�Ӱ�Һ�����ײ���ϡ��ʱҪ��Ũ�������ˮ�У����ܽ�ˮ����Ũ�����У����ò��������Ͻ��裻
�ʴ𰸣���5mL��37mL����ƽ�ţ���Һ�棻��ײ�����ˮ��Ũ�����������
��2�����ĵ�������Һ�����ʵ�����Ϊ��30g��20%=6g
��һ������Ʒ�к��������Ƶ�������x
2NaOH+H2SO4=Na2SO4+2H2O
80 98
x 6g
| 80 |
| x |
| 98 |
| 6g |
x=4.9g
��һ������Ʒ�к��������Ƶ�������4.9g��
������������Ŀ�Ƚϼ��ǻ����ĸ��ݻ�ѧ����ʽ�ļ����⣬�ؼ���Ҫ�������⣬���������ƺ�����������Һ���������������

��ϰ��ϵ�д�
�����Ŀ
��֪�ϻ��ý���������������ˮ��Ӧ������Ӧ�ļ�������ʼ��ÿһת�����ܶ�ͨ��һ����Ӧʵ�ֵ��ǣ�������
| A��CuO��Cu��CuO |
| B��O2��Fe3O4��O2 |
| C��NaOH��H2O��NaOH |
| D��Fe��FeCl2��Fe |
���й��ڱ�����Һ��˵������ȷ���ǣ�������
| A��������Һ�������¶�ʱ��һ���ܼ����ܽ����� |
| B���ı�������ʹ������Һ�Ͳ�������Һ֮���ת�� |
| C���κ����ʵı�����Һ�����¶Ƚ���ʱ��һ������������ |
| D��һ�����ʵı�����Һ�У��������ܽ��������� |

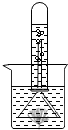 ��ͼ��ʾ����֤��ֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ�װ���뱭ˮ���ձ�����һЩ�����壬��ͨ��һ����������A��ֹһ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ���һ������Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣺
��ͼ��ʾ����֤��ֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ�װ���뱭ˮ���ձ�����һЩ�����壬��ͨ��һ����������A��ֹһ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ���һ������Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣺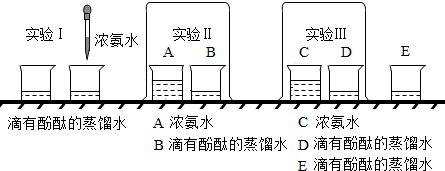
 ������̼��ʵ�����Ʒ�
������̼��ʵ�����Ʒ�