��Ŀ����
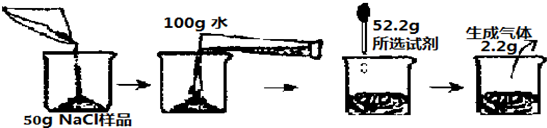
 ��һ�λ�ѧ�����У���ѧ��ʦ��С��ͬѧ30gNaCl������Ʒ������CuCl2���ʣ���Ҫ���ȥ���ʣ��������Ʒ���Ȼ�ͭ������������С����30gNaCl��Ʒ����100gˮ�У�����ȫ���ܽ����������������Һ�����ɵij�����μӵ�����������Һ��������ϵ��ͼ��ʾ��
��һ�λ�ѧ�����У���ѧ��ʦ��С��ͬѧ30gNaCl������Ʒ������CuCl2���ʣ���Ҫ���ȥ���ʣ��������Ʒ���Ȼ�ͭ������������С����30gNaCl��Ʒ����100gˮ�У�����ȫ���ܽ����������������Һ�����ɵij�����μӵ�����������Һ��������ϵ��ͼ��ʾ����1����30gNaCl�������Ȼ�ͭ��������������������ȷ��0.1%��
��2����ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�����������ȷ��0.1%��
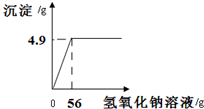
��������1��������������ʿ���֪���������ķ�ӦΪ�Ȼ�ͭ���������Ƶķ�Ӧ������ͼ����Ϣ����֪�����ɳ���������Ϊ2.9g���ó���Ϊ������ͭ������������ͭ��������Ϸ�Ӧ�Ļ�ѧ����ʽ����������Ȼ�ͭ������������������Ȼ�ͭ������������
��2����Ӧ���Ȼ�����Һ��������Դ����Ʒ�к��еĺͷ�Ӧ�����ɵģ���Ӧ����Һ���������Ը��������غ㶨�������㣬����Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������
��2����Ӧ���Ȼ�����Һ��������Դ����Ʒ�к��еĺͷ�Ӧ�����ɵģ���Ӧ����Һ���������Ը��������غ㶨�������㣬����Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������
����⣺��������ͼ�ɵ�Cu��OH��2�������T4.9 g��
���Ȼ�ͭ������Ϊx�����ɵ�NaCl������Ϊy����
CuCl2+2NaOH�T2NaCl+Cu��OH��2��
135 117 98
x y 4.9g
=
=
��ã�x=6.75g��y=5.85g��
��1����Ʒ��CuCl2�����������T
��100%�T22.5%��
��2��NaCl���������T5.85g+30g-6.75g=29.1g��
������Һ��NaCl�����������T
��100%=16.7%��
�𣺣�1����Ʒ���Ȼ�ͭ����������Ϊ22.5%��
��2��������Һ���Ȼ��Ƶ���������Ϊ16.7%��
���Ȼ�ͭ������Ϊx�����ɵ�NaCl������Ϊy����
CuCl2+2NaOH�T2NaCl+Cu��OH��2��
135 117 98
x y 4.9g
| 135 |
| x |
| 117 |
| y |
| 98 |
| 4.9g |
��ã�x=6.75g��y=5.85g��
��1����Ʒ��CuCl2�����������T
| 6.75g |
| 30g |
��2��NaCl���������T5.85g+30g-6.75g=29.1g��
������Һ��NaCl�����������T
| 29.1g |
| 30g+100g+56g-4.9g |
�𣺣�1����Ʒ���Ȼ�ͭ����������Ϊ22.5%��
��2��������Һ���Ȼ��Ƶ���������Ϊ16.7%��
������Ҫ�����������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ�Ȼ���������������龰��ͼ����Ϣ�ȣ������ѧ�����֪ʶ�ͼ��ܣ�������ĿҪ����������ѡ����ɣ�
ע�ⷴӦ����Һ���������㷽�������������غ㶨�������㣬����Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������
ע�ⷴӦ����Һ���������㷽�������������غ㶨�������㣬����Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������

��ϰ��ϵ�д�
�����Ŀ