��Ŀ����
5��ij��ѧ��ȤС���ͬѧΪ�˲ⶨij�������������������е����ʲ����뷴Ӧ�������������������������������ͼ��ʵ��װ�ã��Իش��������⣺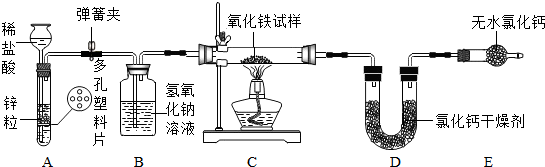
��1��A����װ�õ��ŵ����ܿ��Ʒ�Ӧ�ķ�������ֹ��
��2����C��Ӳ�ʲ����ڷ����Ļ�ѧ����ʽ3H2+Fe2O3$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O��
��3��10�˲�����������������C��Ӳ�ʲ����ڣ��Ƶ�U�ιܺ��Ȼ��Ƹ����������Ϊ23.4�ˣ�������װ�ý���ʵ�飬������ַ�Ӧ��Ƶ�U�ιܺ��Ȼ��Ƹ����������Ϊ26.1�ˣ�������������������������Ϊ80%��
��4��ͬѧ��Ƶ����ʵ�鷽���в�����֮������Ľ����ǿ�����װ��B��C֮�䣬����һ���������װ�ã���ʢ��Ũ�����ϴ��ƿ�����Ա����մ�װ��B�е����������������ˮ���������������
���� ������������ȡ�����ʡ���������ʡ�����ĸ����Ӧ��ԭ����ʵ�鷽����������۵�֪ʶ�������ۺϷ������ƶϺͽ��
��� �⣺��1��A����װ�������շ�������ԭ����ͬ�����ŵ�Ϊ���ܿ��Ʒ�Ӧ�ķ�������ֹ������ܿ��Ʒ�Ӧ�ķ�������ֹ��
��2�����������������ȷ�Ӧ��������ˮ�����3H2+Fe2O3$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O��
��3������ˮ������Ϊ��26.1g-23.4g=2.7g����������������Ϊx
3H2+Fe2O3$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O
160 54
x 2.7g
$\frac{160}{54}=\frac{x}{2.7g}$ x=8g
��������������������$\frac{8g}{10g}��100%$=80%
���80%��
��4��Ϊ�˲��ô�װ��B�е����������������ˮ��������װ��D�����ն�������������װ��B��C֮�䣬����һ���������װ�ã���ʢ��Ũ�����ϴ��ƿ�����Ա����մ�װ��B�е����������������ˮ������������������������װ��B��C֮�䣬����һ���������װ�ã���ʢ��Ũ�����ϴ��ƿ�����Ա����մ�װ��B�е����������������ˮ���������������
���� ���⿼���֪ʶ��϶֪࣬ʶ��Ϲ㣻��һ���Ƚϸ��ӵ��ۺ�ʵ������⣮���ʱ��һ��Ҫϸ�ġ�����ؽ��з���̽���������п�ѧ�������ƶϺ���ơ�����ʵ�鷽���ȣ�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�| A�� | ˤ���˵Ĵ������ƴ����һ��--���Ӽ���ڳ��� | |
| B�� | �Ź�ź���ȵ�ʱ����������--�¶�Խ�ߣ����ӵ��˶�Խ���� | |
| C�� | ǽ�ڿ���ǽ����--�����ڲ�ͣ���������˶� | |
| D�� | ������ҹ�²�仯��ɳĮ��С--ˮ�ı����ݱ�ɳʯ�ı����ݴ� |
| A�� | ��Ũ��������ע��ʢ��ˮ����Ͳ�� | |
| B�� | ������ԭ����ͭʱ���ȼ�������ͭ��ͨ���� | |
| C�� | ʵ�����Ʊ�����ʱ����װ��ҩƷ���ټ���װ�õ������� | |
| D�� | ����������ʱ����ֽ�ı�ԵӦ��©�����Ե� |
ʵ��һ�����ÿ��������������
| ʵ�鲽�� | ���ͻ���� |
| ��1�������½ྻ�Ŀ���ͨ������������Һ����ͨ��Ũ���� | ͨ������������ҺĿ����Ϊ�˿����еĶ�����̼��ͨ��Ũ�����Ŀ����Ϊ�˳�ȥ�����е�ˮ������ |
| ��2��������ͨ�����ȵ�ͭ�� | ��ȥ�����е������� |
| ��3���ռ����壬���ⶨ��������ܶ� | �ܶ�Ϊ1.2572g/�� |
����������ʵ�����ĵ����ܶȲ�һ���������ԭ����Ϊ�����л���������ϡ�����壮
| ���� | ���� | CH4 | CO2 | SO2 | H2 | NH3 |
| ��״���µ��ܶ� | 1.29 | 0.72 | 1.98 | 2.86 | 0.09 | 0.77 |
| ��״����1���ˮ�����ܽ��������� | 0.033 | 0.83 | 40 | 0.018 | 680 |
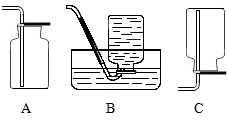
��1������Aװ���ռ���������CO2��SO2��
��2������Bװ���ռ���������CH4��H2��
��3������Cװ���ռ���������CH4��H2��NH3�������ѧʽ��
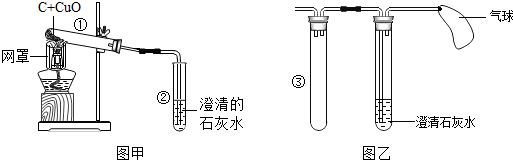
��1���տ�ʼԤ��ʱ���Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ���ǿ�ʼ�ų������Թ��ڵĿ�����
��2���������ȣ��۲쵽�Թܢ��г���ʯ��ˮ����ǣ��Թܢ��к�ɫ��ĩ�г��ֺ�ɫ���ʣ�����д�����е�һ����ѧ����ʽC+2CuO$\frac{\underline{\;����\;}}{\;}$2Cu+CO2����
��3����ַ�Ӧ�۲쵽�Թܢ��е�ʣ���������������������ɫ���壬Ϊ��ȷ���ú�ɫ����ijɷ֣�ͬѧ�ǽ���������̽������������������±���
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ������Ӧ����Թܢ��еĹ����ڽྻ�Թ��ڣ�����ϡ����ϡ���� �ȣ� | A | �ú�ɫ������ľ̿ |
| B | �ú�ɫ����������ͭ |
��4����ͬѧ�����ͼ����ʾ��ʵ��װ�ô��ڲ��㣬�������ͼ����ʾʵ��װ�����ͼ�����Թܢڲ��֣�ͼ����������������ռ�β������ֹһ����̼�Դ�������Ⱦ�ռ�β������ֹһ����̼�Դ�������Ⱦ���Թܢ۵������Ƿ�������
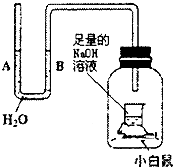 ��ͼ��ij��ȤС����Ƶ�һ��ʵ��װ�ã�װ������ά��ʵ�������С������������ƿ���ܷ⣮װ�����������ã�����Сʱ��u�ι�A��B������Һ���������������������NaOH��Һ�����ն�����̼����������
��ͼ��ij��ȤС����Ƶ�һ��ʵ��װ�ã�װ������ά��ʵ�������С������������ƿ���ܷ⣮װ�����������ã�����Сʱ��u�ι�A��B������Һ���������������������NaOH��Һ�����ն�����̼����������| A�� | A��������B���½� | B�� | A��B�������½� | C�� | A���½���B������ | D�� | A��B���������� |
 ��ͼ��ij�Լ�ƿ��ǩ�IJ������ݣ���ش��������⣺
��ͼ��ij�Լ�ƿ��ǩ�IJ������ݣ���ش��������⣺ ����ijƷ�ƿ�Ȫˮ�������װ�ϲ�������˵����ͼ��ʾ����ش��������⣺
����ijƷ�ƿ�Ȫˮ�������װ�ϲ�������˵����ͼ��ʾ����ش��������⣺