��Ŀ����
2�� Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g������1����Ʒ���������Ƶ�������
��2�����������Ʒ�Ӧ�������������
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ������֪Na2CO3+2HCl�T2NaCl+H2O+CO2����
���� ��1�����÷���ʽNa2CO3+2HCl�T2NaCl+H2O+CO2��������2.2�˶�����̼�������������ȫ��Ӧ��̼���Ƶ�������
��2����ʼʱ����������Ǻ��������Ʒ�Ӧ�������ɶ�����̼������������Һ��Ӧ��Ϻ�������Ǻ�̼���Ʒ�Ӧ����ʱ���ɶ�����̼���н��
��3�����ݻ�ѧ����ʽ�����֪��������������������ɶ�����̼��������һֱ�߷��̣��ݴ˻������ߣ�
��� �⣺��1������Ʒ��̼���Ƶ�����Ϊx��̼�����������������Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y��7.3% 2.2g
$\frac{106}{x}=\frac{73}{7.3%y}=\frac{44}{2.2g}$
x=5.3g
y=50g
��Ʒ���������Ƶ�������13.3g-5.3g=8g
����Ʒ���������Ƶ�����Ϊ8g��
��2������������Ʒ�Ӧ��ϡ���������Ϊz��
NaOH+HCl�TNaCl+H2O
40 36.5
8g z��7.3%
$\frac{40}{8g}=\frac{36.5}{7.3%z}$
z=100g
�𣺺��������Ʒ�Ӧ��ϡ���������Ϊ100g��
��3����ʼ�������ƺ����ᷴӦ���ų�������̼���������������������������Ϊ100g��Ȼ��̼���ƺ����ᷴӦ�ų�������̼������̼�����������������Ϊ50g�����ɶ�����̼������Ϊ2.2g�����Թ�ϵͼΪ��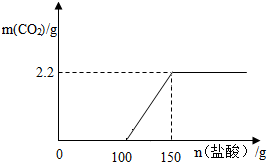 ��
��
���� ����Ŀ��ʵ��ͼ�����һ�壬�ǿ�����ص����ͣ�Ҫ��ѧ�����������Ҫע��֪ʶ�����ϵ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ˮ��������Ŀ��� | |
| B�� | Һ��������������ȫ�ֽ��ʣ��Һ�� | |
| C�� | �����������ơ�� | |
| D�� | ��ˮ����������ؼ��Ⱥ�Ĺ���ʣ���� |
| A�� | ʯī���� | B�� | ����̿����ˮ | ||
| C�� | �õ����� | D�� | ��ʯīת���ɽ��ʯ |
