��Ŀ����
ijʯ�ҳ�Ϊ�˲ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ��4gʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ�У���Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦ��Ҳ������ˮ������ַ�Ӧ���ˡ�����Ȳ���������������ʵ�����������
��1��д����Ӧ�Ļ�ѧ����ʽ ��
��2���г�4gʯ��ʯ��ȫ��Ӧ���ɵĶ�����̼������x���ı���ʽ ��
��3����ϡ�����������������Ϊ ��
��4����ڶ��η�Ӧ���������Һ�м���8.88gˮ�����ò�������Һ��������������Ϊ ��
��5����Ҫ����20g����ϡ������Ҫ36.5%��Ũ�����ˮ��������Ϊ ��
| ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ϡ��������� | 5g | 5g | 5g | 5g |
| ʣ���������� | 3g | 2g | 1g | 1g |
��2���г�4gʯ��ʯ��ȫ��Ӧ���ɵĶ�����̼������x���ı���ʽ
��3����ϡ�����������������Ϊ
��4����ڶ��η�Ӧ���������Һ�м���8.88gˮ�����ò�������Һ��������������Ϊ
��5����Ҫ����20g����ϡ������Ҫ36.5%��Ũ�����ˮ��������Ϊ
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��2������ͼ����֪ÿ5g���ᷴӦ�������1g�����Ĵμ��������������ٸı䣬˵��̼����Ѿ���ȫ��Ӧ�ǣ�ʣ�����������������ʵ��������Ӷ����̼��Ƶ����������÷���ʽ�������������̼��������
��3������ÿ5g������ȫ��Ӧ����̼��Ƶ�����Ϊ1g������̼��Ƶ���������Ȼ�����������������ϡ��������������������ɣ�
��4������̼��Ƶ�������������Ȼ��Ƶ����������������������Һ�����������������ɣ�
��5��������Һϡ���������ʲ�����н��
��2������ͼ����֪ÿ5g���ᷴӦ�������1g�����Ĵμ��������������ٸı䣬˵��̼����Ѿ���ȫ��Ӧ�ǣ�ʣ�����������������ʵ��������Ӷ����̼��Ƶ����������÷���ʽ�������������̼��������
��3������ÿ5g������ȫ��Ӧ����̼��Ƶ�����Ϊ1g������̼��Ƶ���������Ȼ�����������������ϡ��������������������ɣ�
��4������̼��Ƶ�������������Ȼ��Ƶ����������������������Һ�����������������ɣ�
��5��������Һϡ���������ʲ�����н��
����⣺��1�������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2�������CaCO3+2HCl�TCaCl2+H2O+CO2����
��2������ͼ����֪ÿ5g���ᷴӦ�������1g�����Ĵμ��������������ٸı䣬˵��̼����Ѿ���ȫ��Ӧ�ǣ�ʣ�����������������ʵ�������̼��Ƶ�����=4g-1g=3g��
�����ɶ�����̼������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
3g x
=
���
=
��
��3��ÿ5g������ȫ��Ӧ����̼��Ƶ�����Ϊ1g������10g��������̼��Ƶ�����Ϊ2g
��ϡ�������������Ϊy�������Ȼ��Ƶ�����Ϊz�����ɶ�����̼������Ϊw��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
2g y z w
=
=1.46g
=
z=2.22g
=
w=0.88g
��ϡ�����������������=
��100%=14.6%�����14.6%��
��4����ڶ��η�Ӧ���������Һ�м���8.88gˮ�����ò�������Һ��������������=
��100%=11.1%�����11.1%��
��5����36.5%��Ũ���������Ϊn��
20g��14.6%=n��36.5%
n=8g
ˮ������=20g-8g=12g
���Ե�Ũ�����ˮ��������=8g��12g=2��3�����2��3��
��2������ͼ����֪ÿ5g���ᷴӦ�������1g�����Ĵμ��������������ٸı䣬˵��̼����Ѿ���ȫ��Ӧ�ǣ�ʣ�����������������ʵ�������̼��Ƶ�����=4g-1g=3g��
�����ɶ�����̼������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
3g x
| 100 |
| 3g |
| 44 |
| x |
���
| 100 |
| 3g |
| 44 |
| x |
��3��ÿ5g������ȫ��Ӧ����̼��Ƶ�����Ϊ1g������10g��������̼��Ƶ�����Ϊ2g
��ϡ�������������Ϊy�������Ȼ��Ƶ�����Ϊz�����ɶ�����̼������Ϊw��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
2g y z w
| 100 |
| 2g |
| 73 |
| y |
=1.46g
| 100 |
| 2g |
| 111 |
| z |
z=2.22g
| 100 |
| 2g |
| 44 |
| w |
w=0.88g
��ϡ�����������������=
| 1.46g |
| 10g |
��4����ڶ��η�Ӧ���������Һ�м���8.88gˮ�����ò�������Һ��������������=
| 2.22g |
| 10g+2g-0.88g+8.88g |
��5����36.5%��Ũ���������Ϊn��
20g��14.6%=n��36.5%
n=8g
ˮ������=20g-8g=12g
���Ե�Ũ�����ˮ��������=8g��12g=2��3�����2��3��
������������Ҫ����ѧ������ͼ�����ݣ����û�ѧ����ʽ���м����������

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ
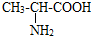 �������ǹ��ɵ����ʵĻ�����λ�����Ļ�ѧʽ��C3H702N�����й��ڱ������������ȷ���ǣ�������
�������ǹ��ɵ����ʵĻ�����λ�����Ļ�ѧʽ��C3H702N�����й��ڱ������������ȷ���ǣ�������| A����������̼���⡢����������ԭ�ӹ��� |
| B�������������̼Ԫ������Ԫ�ص�������Ϊ9��8 |
| C��һ������������к���48������ |
| D������������Ԫ�ص������������ |