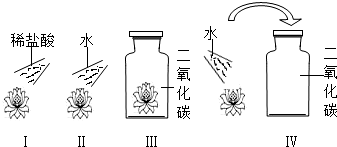
��Ŀ����
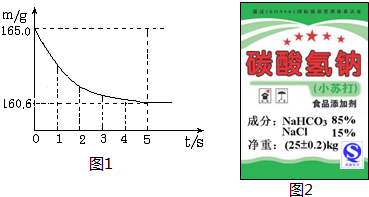
Ϊ�ⶨij����С�մ���NaHCO3������������ʶ�Ƿ���ʵ��ij��ȤС��ȡ10.0gС�մ���Ʒ�����ձ����ټ���������ϡ������ȫ��Ӧ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������Ӧ�ķ���ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2����
���㣺
��1����ȫ��Ӧʱ����������̼������Ϊ g��
��2������˵����С�մ���NaHCO3������������ʶ�Ƿ���ʵ��

���㣺
��1����ȫ��Ӧʱ����������̼������Ϊ
��2������˵����С�մ���NaHCO3������������ʶ�Ƿ���ʵ��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1���Ȼ��ƺ�̼�����ƵĹ���������ϡ�����Ϻ�̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ڷ�Ӧ�ų��˶�����̼�����Է�Ӧ���ձ���ʣ�����ʵ�������С����С��������Ϊ���������̼��������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������̼�������ɼ���ԭ�������̼�����Ƶ����������ɽ��
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������̼�������ɼ���ԭ�������̼�����Ƶ����������ɽ��
����⣺��1�����������غ㶨�ɣ���Ӧ���ɶ�����̼���������=165.0g-160.6g=4.4 g
��2����ԭ�������̼�����Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
NaHCO3+HCl�TNaCl+H2O+CO2��
84 44
x 4.4g
=
��֮�ã�x=8.4g��
��С�մ���NaHCO3����������Ϊ
��100%=84%��84%��85%���ʸ�С�մ���NaHCO3������������ʶ����ʵ��
�𰸣���1��4.4��
��2����С�մ���NaHCO3������������ʶ����ʵ��
��2����ԭ�������̼�����Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
NaHCO3+HCl�TNaCl+H2O+CO2��
84 44
x 4.4g
| 84 |
| x |
| 44 |
| 4.4g |
��֮�ã�x=8.4g��
��С�մ���NaHCO3����������Ϊ
| 8.4g |
| 10.0g |
�𰸣���1��4.4��
��2����С�մ���NaHCO3������������ʶ����ʵ��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м��������������ʱҪ�ر�ע����ⲽ��Ĺ淶�ԣ��Ӷ�����ͨ����֪����δ֪�Ľ��ⷽ����

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д�
�����Ŀ
����������ɿɱ�ʾΪAl2Si2Ox��OH��y������x��y����ֵ�ֱ��ǣ�������
| A��7��2 | B��5��4 |
| C��6��3 | D��3��6 |