��Ŀ����
19��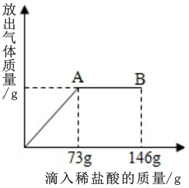 ��һ�ձ���ʢ����Na2CO3��NaCl ��ɵĹ�������25g���������μ�������������Ϊ10%��ϡ���ᣬ�ų���������������ϡ�����������ϵ��ͼ��ʾ����֪��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O�������������ͼ��ش��������⣺
��һ�ձ���ʢ����Na2CO3��NaCl ��ɵĹ�������25g���������μ�������������Ϊ10%��ϡ���ᣬ�ų���������������ϡ�����������ϵ��ͼ��ʾ����֪��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O�������������ͼ��ش��������⣺��1�����μ�ϡ������ͼ��A��ʱ���ձ�����Һ��pH=7
���������=������������Һ�е�����ΪNaCl
���ѧʽ�������μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH�� 7���������=����������
��2��A��ʱ��������HCl������Ϊ7.3g���ų�CO2������4.4��B��ų�CO2������Ϊ4.4 g��
��3������ԭ�����������Ȼ��Ƶ�����������
���� ���ڸ��������ĵ���������������������������Ը���������HCl�������Ͷ�Ӧ�Ļ�ѧ����ʽ�����Ӧ��̼���Ƶ����������ɵ��Ȼ��Ƶ����������������������������������μӹ��̵õ���ͼ�����������μ����ᵽ73gʱΪǡ����ȫ��Ӧ���ӵ�146gʱΪ�����������ʱpHС��7��
��� �⣺����ͼ���Կ��������μ�������73gʱҲ����A��ʱ����ʱ����ﵽ�������Ҳ����ǡ����ȫ��Ӧ����ʱ��ҺpH����7������ֻ���Ȼ��ƣ��������μ�����ʱ����������ʣ�࣬���Դ�ʱpHС��7������ΪHCl��NaCl��
��A��ʱ���ĵ�HCl������Ϊ73g��10%=7.3g��
������7.3gHClʱ��Ӧ��̼���Ƶ�����Ϊx�����ɵĶ�����̼������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x 7.3g y
$\frac{106}{x}$=$\frac{73}{7.3g}$=$\frac{44}{y}$
x�T10.6g
y=4.4g
����B���������̼������Ҳ������4.4g��
���Ի�������Ȼ��Ƶ�����Ϊ25g-10.6g�T14.4g
������� �Ȼ��Ƶ���������Ϊ$\frac{14.4g}{25g}$��100%�T57.6%
�ʴ�Ϊ����1��=�� NaCl������
��2��7.3�� 4.4�� 4.4��
��3��57.6%��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������

| A�� |  ���� | B�� |  ����Һ�� | ||
| C�� |  ��ȥCO�е�ˮ���� | D�� |  ��ȡ9.3mLҺ�� |
| ѡ�� | A | B | C | D |
| ���� | ���� | �� | �� �� | ˮ�� |
| ��������������pH | 5.0��5.5 | 6.0��6.8 | 7.2��8.5 | 6.0��7.0 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� |  �Ͻ��Ŵ� | B�� |  ����ƿ | C�� |  �������� | D�� |  ������̥ |
| A�� | +1 | B�� | +2 | C�� | +3 | D�� | +4 |
| A�� | ������̼ | B�� | ϡ������ | C�� | ���� | D�� | ���� |
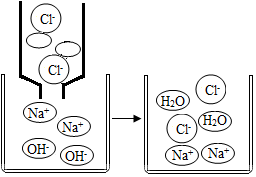 ͼΪ����������Һ��ϡ����ǡ����ȫ��Ӧ����ʾ��ͼ���Իش��������⣺
ͼΪ����������Һ��ϡ����ǡ����ȫ��Ӧ����ʾ��ͼ���Իش��������⣺