��Ŀ����
ˮ������֮Դ����Ȼ���е�ˮ������ͬ��������;���ӹ㷺��

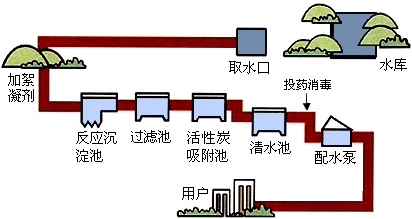
��1����ͼ������ˮ���ľ�ˮ���̣����г�ȥˮ�в��������ʵ���Ҫ�豸��______�أ���ȥ��ζ��һЩ���������ʵ��豸��______�أ��������ϴ���������ˮ���Ǵ�ˮ����Ҫ��ȡ��ˮ��Ӧ��ȡ�ľ���������______��
��2��Ŀǰ����������ˮ��������������ɱ�������������£���������ˮ��Ӧ��������ʹ����ᣨHClO������������ɱ��������
��������ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
����������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�������������Ӱ�����______������ţ���
A��ϡ���� B��̼������Һ C������������Һ D���������Һ��

��1����ͼ������ˮ���ľ�ˮ���̣����г�ȥˮ�в��������ʵ���Ҫ�豸��______�أ���ȥ��ζ��һЩ���������ʵ��豸��______�أ��������ϴ���������ˮ���Ǵ�ˮ����Ҫ��ȡ��ˮ��Ӧ��ȡ�ľ���������______��
��2��Ŀǰ����������ˮ��������������ɱ�������������£���������ˮ��Ӧ��������ʹ����ᣨHClO������������ɱ��������
��������ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
����������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�������������Ӱ�����______������ţ���
A��ϡ���� B��̼������Һ C������������Һ D���������Һ��
��1�������ܳ�ȥˮ�еIJ����Թ��壬�ʳ�ȥˮ�в��������ʵ���Ҫ�豸�ǹ��˳أ�����̿���������ã��ɳ�ȥˮ�в��ֿ��������ʡ���ζ��ɫ�أ��ʳ�ȥ��ζ��һЩ���������ʵ��豸�ǻ���̿�����أ���ȡ��ˮ�ķ�����Ҫͨ������ķ�����
��2����������ˮ��Ӧ��������ʹ����ᣨHClO������Ӧ�Ļ�ѧ����ʽΪCl2+H2O�THCl+HClO��
��A����������ˮ�к������ᣬ��ʹ������ˮ���Ƶ�ϡ�����������ˮ�к��������Ӱ�죬��������Ҫ���Ƶ�1%��
B������̼���ƻ�������ˮ�е����ᡢ�����ᷢ����Ӧ�����٣���ʹ������ˮ���Ƶ�̼������Һ����������С����Ҫ���Ƶ�1%��
C�������������ƻ�������ˮ�е����ᡢ�����ᷢ����Ӧ�����٣���ʹ������ˮ���Ƶ�����������Һ����������С����Ҫ���Ƶ�1%��
D����������ز���������ˮ�е����ᡢ������ȷ�����Ӧ����ʹ������ˮ���Ƶ��������Һ���������������ܵ�����ˮ�����ʵ�Ӱ�죮
��ѡD��
�ʴ�Ϊ����1�����ˣ�����̿����������
��2����Cl2+H2O�THCl+HClO����D��
��2����������ˮ��Ӧ��������ʹ����ᣨHClO������Ӧ�Ļ�ѧ����ʽΪCl2+H2O�THCl+HClO��
��A����������ˮ�к������ᣬ��ʹ������ˮ���Ƶ�ϡ�����������ˮ�к��������Ӱ�죬��������Ҫ���Ƶ�1%��
B������̼���ƻ�������ˮ�е����ᡢ�����ᷢ����Ӧ�����٣���ʹ������ˮ���Ƶ�̼������Һ����������С����Ҫ���Ƶ�1%��
C�������������ƻ�������ˮ�е����ᡢ�����ᷢ����Ӧ�����٣���ʹ������ˮ���Ƶ�����������Һ����������С����Ҫ���Ƶ�1%��
D����������ز���������ˮ�е����ᡢ������ȷ�����Ӧ����ʹ������ˮ���Ƶ��������Һ���������������ܵ�����ˮ�����ʵ�Ӱ�죮
��ѡD��
�ʴ�Ϊ����1�����ˣ�����̿����������
��2����Cl2+H2O�THCl+HClO����D��

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
�����Ŀ