��Ŀ����
ˮ������֮Դ����Ȼ���ˮ������ͬ��������;���ӹ㷺��
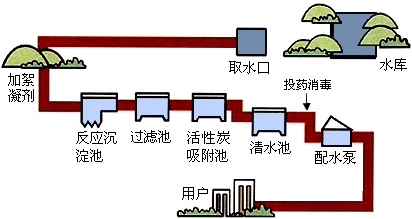
��1����ͼ������ˮ���ľ�ˮ���̣����г�ȥ��ζ���豸��
��2��Ŀǰ����������ˮ��������������ɱ�������������£���������ˮ��Ӧ��������ʹ����ᣨHClO������������ɱ��������
��������ˮ��Ӧ�Ļ�ѧ����ʽΪ
����������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�������������Ӱ�����
A��ϡ���� B��̼������Һ C������������Һ D���������Һ��

��1����ͼ������ˮ���ľ�ˮ���̣����г�ȥ��ζ���豸��
����̿����
����̿����
�أ���2��Ŀǰ����������ˮ��������������ɱ�������������£���������ˮ��Ӧ��������ʹ����ᣨHClO������������ɱ��������
��������ˮ��Ӧ�Ļ�ѧ����ʽΪ
Cl2+H2O�THCl+HClO
Cl2+H2O�THCl+HClO
������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�������������Ӱ�����
D
D
������ţ���A��ϡ���� B��̼������Һ C������������Һ D���������Һ��
��������1�����ݻ���̿�������Է�����
��2���ٸ����ڳ�������������ˮ��Ӧ��������ʹ����ᣨHClO����д����Ӧ�ķ���ʽ��
�ڸ�������ˮ�к��е����ʼ������ʷ����жϣ�
��2���ٸ����ڳ�������������ˮ��Ӧ��������ʹ����ᣨHClO����д����Ӧ�ķ���ʽ��
�ڸ�������ˮ�к��е����ʼ������ʷ����жϣ�
����⣺��1�����ڻ���̿�������ԣ�������һЩɫ�غ���ζ�����ԣ�����ˮ���ľ�ˮ���̣����г�ȥ��ζ���豸�ǻ���̿������
��2������������ˮ��Ӧ��������ʹ����ᣨHClO������Ӧ�ķ���ʽ�ǣ�Cl2+H2O�THCl+HClO��
��A����������ˮ�к���ϡ���ᣬ������������������Ϊ1%��ϡ������Ӱ�죮��A���������⣻
B����������ˮ�к��е�ϡ����ʹ���������̼������Һ��Ӧ��������������������Ϊ1%��̼������Һ��Ӱ�죮��B���������⣻
C����������ˮ�к���ϡ����ʹ�������������������Һ��Ӧ��������������������Ϊ1%������������Һ��Ӱ�죮��C���������⣻
D����������ˮ�к��еijɷֲ����������Һ��Ӧ��������������������Ϊ1%���������Һ��Ӱ�죮��D�������⣮
�ʴ�Ϊ����1������̿�����أ� ��2����Cl2+H2O�THCl+HClO�� ��D��
��2������������ˮ��Ӧ��������ʹ����ᣨHClO������Ӧ�ķ���ʽ�ǣ�Cl2+H2O�THCl+HClO��
��A����������ˮ�к���ϡ���ᣬ������������������Ϊ1%��ϡ������Ӱ�죮��A���������⣻
B����������ˮ�к��е�ϡ����ʹ���������̼������Һ��Ӧ��������������������Ϊ1%��̼������Һ��Ӱ�죮��B���������⣻
C����������ˮ�к���ϡ����ʹ�������������������Һ��Ӧ��������������������Ϊ1%������������Һ��Ӱ�죮��C���������⣻
D����������ˮ�к��еijɷֲ����������Һ��Ӧ��������������������Ϊ1%���������Һ��Ӱ�죮��D�������⣮
�ʴ�Ϊ����1������̿�����أ� ��2����Cl2+H2O�THCl+HClO�� ��D��
������������ѶȲ�����������ˮ����ˮ��֪ʶ����Ļ�ѧ���ʡ���ѧ����ʽ����д������������ѧ��֪ʶ�������ķ����ǽ����Ĺؼ���

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ